Allergen Testing - CAM 051
Description:
Allergic disease is characterized by inappropriate or exaggerated rated immune reactions to foreign antigens (allergens) that are generally innocuous to most people, but when introduced into a genetically-predisposed individual, elicit a hypersensitivity reaction (Hamilton, 2021). Hypersensitivity reactions can be classified into four types, two of which are associated with allergy, type I immediate immunoglobulin E (IgE) reactions and type IV T cell mediated reactions (K.-L. Chang & J. C. Guarderas, 2018). Type I reactions involve the formation of IgE antibodies specific to the allergen. When the subject is re-exposed to that allergen, the allergen binds multiple IgE molecules, resulting in the release of an array of inflammatory mediators, including histamines, that precipitate the symptoms of allergic disease (Hamilton, 2021).
Allergen testing in serum is designed to detect the presence of allergen specific IgE. A positive test for allergen specific IgE confirms the presence of the antibody only. Actual reactivity must be determined by history or supervised challenge (Kowal & DuBuske, 2022). Several diagnostic procedures have been developed to elicit and assess hypersensitivity reactions including epicutaneous, intradermal, patch, bronchial, exercise, and ingestion challenge tests (Bernstein et al., 2008).
Regulatory Status
The FDA has cleared 44 assays for total IgE as of July 13, 2021 (FDA, 2021). Additionally, many labs have developed specific tests that they must validate and perform in house. These laboratory-developed tests (LDTs) are regulated by the Centers for Medicare & Medicaid Services (CMS) as high-complexity tests under the Clinical Laboratory Improvement Amendments of 1988 (CLIA ’88). FDA clearance or approval is not currently required for clinical use.
Allergy testing for CMS plans may be covered when Medicare coverage criteria are met. Refer to the Medicare Claims Processing Manual Chapter 12 — Physicians/Nonphysician Practitioners for details. In addition, Local Coverage Determinations (LCDs)/Local Coverage Articles (LCAs) are available for reference. Compliance with these policies is mandatory where applicable. Find these LCDs/LCAs at https://www.cms.gov/medicare-coverage-database/new-search/search.aspx (CMS, 2021).
Policy:
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
- Specific IgE in-vitro allergy testing is considered MEDICALLY NECESSARY in any of the following situations:
- In lieu of skin testing for an initial allergy screen
- When skin testing is contraindicated (see Note 1)
- When further treatment decisions would be impacted by confirmation of sensitivity in individuals for whom direct skin testing results are not consistent with the history of an anaphylactic or other severe reaction to an allergen
- When limited to allergens chosen for testing based on an individual’s history, physical examination, and environment, specific IgE in-vitro allergy testing (up to 20 allergen specific antibodies per year) is considered MEDICALLY NECESSARY.
- In-vitro testing for total serum IgE is considered MEDICALLY NECESSARY in any of the following situations:
- For individuals with moderate to severe asthma who are being considered for Xolair therapy
- For individuals who are suspected of having allergic bronchopulmonary aspergillosis
- To monitor for allergy resolution in children and adolescents with an initial positive food allergen result(s), annual re-testing for the same food allergen(s) is considered MEDICALLY NECESSARY.
- In the absence of a new clinical presentation, routine re-testing for allergies to the same allergens (except where specified above) is considered NOT MEDICALLY NECESSARY.
- The antigen leukocyte antibody test (ALCAT) is considered NOT MEDICALLY NECESSARY.
The following does not meet coverage criteria due to a lack of available published scientific literature confirming that the test(s) is/are required and beneficial for the diagnosis and treatment of a patient’s illness.
- For the evaluation of a suspected allergy, in-vitro testing of IgG, IgA, IgM, and/or IgD is considered NOT MEDICALLY NECESSARY.
- To measure hypersensitivity to allergens, basophil activation flow cytometry testing (BAT) is considered NOT MEDICALLY NECESSARY.
- In-vitro allergen testing using bead-based epitope assays (e.g., VeriMAP Peanut Dx) is considered NOT MEDICALLY NECESSARY.
- For all situations, in-vitro testing using qualitative specific IgE multi-allergen screen that does not identify a specific allergen is considered NOT MEDICALLY NECESSARY.
NOTES:
Note 1: Skin testing is contraindicated in the following situations:
- Patients who have certain skin conditions (e.g., dermatographism, urticaria, cutaneous mastocytosis, atopic dermatitis, severe diffuse psoriasis)
- Patients who are taking medications that may interfere with the treatment of anaphylaxis (e.g., Beta-blockers and Angiotensin Converting Enzyme inhibitors) or may impair skin test sensitivity (e.g., tricyclic antidepressants, antihistamines)
- Patients who are at high risk to testing (e.g., poorly controlled asthma, clinical history of severe reaction to minute amounts of allergen, cardiac arrhythmia, unstable angina)
- Patients who have experienced an anaphylactic event within the past one month
- Uncooperative patients (e.g., small children, individuals with mental or physical impairments)
Policy Guidelines
Skin testing is contraindicated in the following situations:
- Patients who have certain skin conditions (e.g., dermatographism, urticaria, cutaneous mastocytosis, atopic dermatitis, severe diffuse psoriasis)
- Patient who are taking medications that may interfere with the treatment of anaphylaxis (e.g., Beta-blockers and Angiotensin Converting Enzyme inhibitors) or may impair skin test sensitivity (e.g., tricyclic antidepressants, antihistamines)
- Patients who are at high risk to testing (e.g., poorly controlled asthma, clinical history of severe reaction to minute amounts of allergen, cardiac arrhythmia, unstable angina)
- Patients who have experienced an anaphylactic event within the past one month
- Uncooperative patients (e.g., small children, individuals with mental or physical impairments)
Table of Terminology
|
Term |
Definition |
|
AAAAI |
The American Academy of Allergy, Asthma, and Immunology |
|
AAAI |
Academy of Allergy, Asthma, and Immunology |
|
AAFP |
American Academy of Family Physicians |
|
AAP |
American Academy of Pediatrics |
|
ACAAI |
American College of Allergy, Asthma, and Immunology |
|
ACD |
Allergic contact dermatitis |
|
ACR |
American cockroach |
|
AIT |
Allergen immunotherapy |
|
ALCAT |
The Antigen Leukocyte Antibody Test |
|
AR |
Allergic rhinitis |
|
Arah2-sIgE |
Arachis hypogaea 2-specific IgE |
|
ARIA |
Allergic Rhinitis and its Impact on Asthma |
|
ATP |
Atopy patch test |
|
AUC |
Area under the curve |
|
BAT |
Basophil activation flow cytometry testing |
|
BAT |
Basophil activation test |
|
BBEA |
Peanut bead-based epitope assay |
|
CAA |
Current allergic asthma |
|
CAR |
Current allergic rhinitis |
|
CBS |
Consensus based statements |
|
CD4+ |
Cluster of differentiation 4 |
|
CLIA ’88 |
Clinical Laboratory Improvement Amendments of 1988 |
|
CLSI |
Clinical and Laboratory Standards Institute |
|
CMS |
Centers for Medicare & Medicaid Services |
|
CRS |
Chronic rhinosinusitis |
|
CW |
Choosing wisely |
|
EAACI |
European Academy of Allergy and Clinical Immunology |
|
EDTA |
Ethylenediaminetetraacetic Acid |
|
EP |
Expert panel |
|
FA |
Food allergy |
|
FcεRI |
High-affinity IgE receptor |
|
FDA |
The Food and Drug Administration |
|
FEV1 |
Forced expiratory volume in 1 second |
|
GA2LEN |
Global Allergy and Asthma European Network |
|
H1 |
H-1 receptor antagonists |
|
H2 |
H-2 receptor antagonists |
|
IgA |
Immunoglobulin A |
|
IgD |
Immunoglobulin D |
|
IgE |
Immunoglobulin E |
|
IgE-FAB |
Immunoglobulin E- fragment antigen-binding region |
|
IgG |
Immunoglobulin G |
|
IgG4 |
Immunoglobulin A |
|
IgM |
Immunoglobulin A |
|
JTFPP |
Joint Task Force on Practice Parameters |
|
LCDs |
Local coverage determinations |
|
LDTs |
Laboratory-developed tests |
|
LEAP |
Learning early about peanut allergy |
|
MBB |
Mucosal brush biopsies |
|
MFI |
Median fluorescence intensity |
|
NASEM |
The National Academies of Science, Engineering and Medicine |
|
NIAID |
National Institute of Allergy and Infectious Diseases |
|
NICE |
National Institute for Health and Care Excellence |
|
NPV |
Negative predictive value |
|
NSAIDs |
Nonsteroidal anti-inflammatory drugs |
|
OFC |
Oral food challenge |
|
PAMD@ |
Precision allergy molecular diagnostic applications |
|
PPV |
Positive predictive value |
|
RARS |
Recurrent acute rhinosinusitis |
|
SAR |
Seasonal allergic rhinitis |
|
sIgE |
Specific immunoglobulin E |
|
s-IgE |
Specific immunoglobulin E |
|
SPT |
Skin prick tests |
|
SSRIs |
Selective serotonin reuptake inhibitors |
|
ST |
Skin test |
|
Th2 |
T helper type 2 |
|
tIgE |
Total immunoglobulin E |
|
WAO |
World Allergy Organization |
Rationale
Allergies affect over 50 million Americans, including approximately 30 percent of adults and 40 percent of children (Jackson et al., 2013; NASEM, 2016). The incidence of allergic disease is increasing (Pawankar et al., 2013) and is estimated to result in over $17 billion in health care costs and 200,000 emergency department visits annually (Adams et al., 2013).
A majority of environmental, food, and medication allergies with clinical significance are type I immunoglobulin E (IgE)-mediated allergies (Kowal & DuBuske, 2022). Diagnosis of an IgE-mediated allergy involves identification of the allergen, demonstration of IgE specific to that allergen, and confirmation that symptoms occur when the patient is exposed to the allergen. The IgE response to an allergen can be assessed using skin or serum testing. Patch testing is preferred for delayed T-cell mediated response (K.-L. Chang & J. C. Guarderas, 2018; Zug et al., 2014).
Allergic diseases, respiratory infections, and autoimmune conditions have similar clinical presentations and self-reported symptoms have a relatively low positive predictive value (PPV) (Sampson et al., 2014). Thus, laboratory allergy and immunologic testing are useful in clarifying diagnosis and guiding treatment when the frequency, duration, and sequelae of upper respiratory infections exceed the norm or when rhinosinusitis or asthma symptoms persist despite treatment (Chow et al., 2012). Allergy testing is also useful in identifying causative allergen in atopic dermatitis (eczema), contact dermatitis, urticaria, angioedema, and food or drug allergies. Knowing the causal allergen helps provide clinically relevant information for avoidance and treatment (K.-L. Chang & J. C. Guarderas, 2018).
Skin Testing
Skin testing is the most rapid, sensitive, and cost-effective testing modality for the detection of immunoglobulin E (IgE)-mediated disease. The procedure lasts less than an hour with minimal patient discomfort. There are several published practice parameters for allergen skin testing (Bernstein et al., 2008; K.-L. Chang & J. C. Guarderas, 2018; Kowal & DuBuske, 2022).
Serum IgE
IgE is one of five immunoglobulins and the one primarily involved in allergic disease. At the cellular level, the allergic response starts with “atopy,” a genetic predisposition to produce specific IgE after exposure to allergens. CD4+ helper T cells are predisposed to the “T helper type 2” (Th2) response, which causes the Th2 cells to secrete large amounts of interleukins 4 and 13, which then promotes production of the allergen specific IgE. From there, the allergen-specific IgE binds to high-affinity receptors on mast cells and basophils. At this point, if the relevant allergen is ingested in large enough amounts, the IgE molecules may cluster (cross-linking). This cross-linking causes the mast cells and basophils to release chemical and protein mediators, resulting in the characteristic allergic response (Stokes & Casale, 2022).
Immunoassays measuring both total IgE and allergen specific IgE in serum and other bodily fluids have been developed. Specific IgE immunoassays do not require patient cooperation, are not limited in patients with skin disease, are not blocked by antihistamines, and pose no risk of adverse reactions (Bernstein et al., 2008; K.-L. Chang & J. C. Guarderas, 2018; Kowal & DuBuske, 2021; Stokes & Casale, 2022). Total IgE is usually unrelated to IgE levels for a specific allergen but may be useful in other conditions, such as asthma (Stokes & Casale, 2022).
Other testing
Patch testing is the gold standard for identification of a contact allergen (Mowad, 2006; Rietschel, 1997). Although occlusive patch testing is the most common technique, open, prophetic (provocative), repeated insult, photopatch, and atopy patch tests are also available if special situations indicate their use (Bernstein et al., 2008).
Cellular activation assays measuring the release of histamine from basophils (Kim et al., 2016; Santos & Lack, 2016) or mast cells (Bahri et al., 2018) as diagnostic or prognostic indicators of allergy have been the subject of intense research. Basophil and eosinophilic reactivity tests have been found to be associated with food-induced allergic responses and have been shown in current research to be modified over time during immunotherapy (Sampson et al., 2014). In particular, the basophil activation test (BAT) has emerged as having superior specificity and comparable sensitivity to diagnose food allergies when compared with skin prick test and specific IgE (Santos & Shreffler, 2017). Histamine release from leukocytes of allergic persons is an excellent in vitro correlate of allergy; however, it is currently still considered a research test by the Academy of Allergy, Asthma, and Immunology (AAAI) (Bernstein et al., 2008).
BAT has the potential to be a useful tool for measuring hypersensitivity to allergens, especially for patients who are not suitable for skin testing due to skin status or prior severe reactions since it is an ex vivo, flow cytometry-based assay. BAT, for use as standard clinical practice, is currently limited by its lack of standardization in methodology as well as between systems used. A study by Depince-Berger et al. (2017) has proposed standardization between systems and instruments using whole blood-ethylenediaminetetraacetic acid (EDTA) samples with instrumentation standardization. “BAT would strongly benefit from easy implementation [EDTA, one step stimulation/labeling, wash, full sample analysis over time parameter, B cell relative basophil count] and standardization of instrument settings on MFI [median fluorescence intensity] targets whatever system or instrument is used” (Depince-Berger et al., 2017). Hemmings et al. (2018) note that standardization, quality assurance, and clinical validation will facilitate the transition of the BAT from research to clinical practice.
Proprietary testing
The Antigen Leukocyte Antibody Test (ALCAT) is another test available for the assessment of allergens. ALCAT measures food/immune reactions through stimulation of leukocytes. The immunological reactions to this stimulation are intended to identify sensitivities regardless of pathway as antibodies do not necessarily need to be involved. CellScienceSystems suggests individuals with a variety of disorders (such as gastrointestinal, neurological, et al.) to take this test (CellScienceSystems, 2021). Although the ALCAT machine is FDA registered and there are a few papers published, results are not reproducible when subject to rigorous testing and do not correlate with clinical evidence of allergy (Beyer & Teuber, 2005; Hammond & Lieberman, 2018; Wuthrich, 2005).
Panels encompassing a large number of analytes are also offered by labs. For example, Genova Diagnostics offers a blood test for IgG and IgE antibodies for 87 different foods. Genova also offers several variations on this test, such as “Vegetarian” (21 foods), “Spices” (23 spices), “Molds” (15 molds), and more (Genova, 2021).
Spiriplex offers a microarray-style panel for allergen testing, called “Allergenex.” This test contains many purified allergen proteins to which a patient’s blood sample can bind. This binding creates a quantifiable signal that allows the user to identify the number of IgE antibodies present, and therefore provide a picture of allergy. Spiriplex offers a test for 28 common food allergens, a test for 40 inhalant allergens, and 68 combined food and inhalant allergens (Spiriplex, 2021).
The VeriMAP Peanut Dx and the VeriMAP™ Peanut Sensitivity are both peanut-allergen specific bead-based epitope assays manufactured by AllerGenis LLC. According to Allergenis, VeriMAP™ has a “95% positive predictive value and can reduce overdiagnosis and anxiety by minimizing false positives” (BioSpace, 2021). This is an emerging technology and additional peer-reviewed literature establishing the analytical validity, clinical validity, and clinical utility of such testing will be further required.
Analytical Validity
Variables that can influence the wheal size when performing skin prick tests (SPT) include multiple operators, extract concentrations and quality, skin test devices, time of day, location on the skin, and the measuring of results (Nelson, 2001; Werther et al., 2012).
In 2006, Oppenheimer and Nelson evaluated variability and analytical validity of skin testing. A questionnaire was sent to all physician and fellow members of the American College of Allergy, Asthma and Immunology who were currently practicing in the United States. The objective of this questionnaire was to determine the diversity of skin testing practices among allergists. The results showed great variability among physicians. In particular, “The average number of skin prick tests performed ranged from 5.09 (grasses) to 10.9 (trees), whereas the average number of intradermal tests performed ranged from 2.03 (grasses) to 5.6 (perennial). The allergen extract concentrations used for intradermal testing varied widely. Expressed as a dilution of the concentrated extracts, 20.8% use 1:100 dilutions, 10.3% use 1:500 dilutions, and 59.4% use 1:1,000 dilutions. Significant variability also occurred regarding devices and the technique with which the devices were used. Most clinicians (92.1%) used the most concentrated extract available for skin prick testing. For reporting the results of skin testing, 53.8% used a 0 to 4+ scale, and only 28.3% measured orthogonal diameters. Of those using a 0 to 4+ scale, two thirds related the results to the size of the histamine control (Oppenheimer & Nelson, 2006).” The results from this survey emphasize potential areas of improvement for allergists regarding skin test use and data.
The Clinical and Laboratory Standards Institute (CLSI) has evaluated the analytical validity of serum IgE measurements and found that “Clinical/diagnostic sensitivity and specificity of IgE antibody assays cannot be accurately determined due to the absence of definitive gold standard methods for defining allergic disease. Total and allergen-specific IgE analyses achieve among the highest analytical performance of any antibody assay by following consensus procedures in CLSI-ILA20-A3” (Hamilton et al., 2015).
Knight et al. (2018) “examined the qualitative concordance between SPT and sIgE as measured on the HYTEC™288 platform for 10 commonly encountered inhalant allergens”; a total of 232 subjects were included. Overall concordance between SPT and sIgE was > 70% for all allergens tested. Sensitivity ranged from 25% to 95% depending on the allergen, while specificity was significantly higher for all allergens (78 – 97%). Negative predictive value (NPV) was > 85% for all allergens tested, while PPV was more variable, ranging from 22% to 88%. The authors noted that “these results are similar to findings in other studies comparing SPT with sIgE” (Knight et al., 2018).
Carlsson et al. (2015) examined the inter- and intra- variability of IgE and IgE receptor expression in the blood of seasonal allergic rhinitis (SAR) subjects. Thirty-two patients with SAR were included; the high-affinity IgE receptor, also known as FcεRI, and the low affinity receptor, also known as CD23, were measured. The authors found that “FcεRI expression on basophils and CD23 expression on B cells showed low intrasubject variability both in and out of the pollen season,” although there was a small seasonal difference with lower total IgE levels and FcεRI expression during the pollen season (Carlsson et al., 2015).
Siroux et al. (2017) explored the effect of allergen nature, route of exposure, and dose of exposure on IgE and IgG responses. A total of 340 patients (170 with asthma, 170 without) were included, and IgE/IgG responses to 47 inhalant and food allergens were analyzed and compared between 5 French regions according to route of allergen exposure (inhaled or food). “Ubiquitous” allergens (grass, olive/ash pollen, house dust mites) did not show marked difference in specific IgE level between regions. For region-specific allergens (ragweed, birch, cypress), IgE sensitization was associated with regional pollen exposure. Airborne allergens cross-reacting with food allergens led to frequent IgG recognition. The authors concluded that “the variability in allergen-specific IgE and IgG frequencies depends on exposure, route of exposure, and overall immunogenicity of the allergen. Allergen contact by the oral route might preferentially induce IgG responses” (Siroux et al., 2017).
Sookrung et al. (2019) measured the agreement of a SPT and serum specific IgE test to Periplaneta americana (American cockroach, ACR) allergies. ACR-extract was used, and sera was obtained from 66 individuals clinically diagnosed with chronic allergic rhinitis. Of the 66 samples, 46 were positive and 20 negative after a SPT to ACR-extract. Serum IgE levels were then measured by a commercial test kit. The authors note that of the SPT positive cases to ACR-extract, only 32.6% were also positive for serum IgE, indicating low concordance between the two testing methods (Sookrung et al., 2019).
He and Reisacher (2019) measured the sensitivity, specificity, and predictive value of oral mucosal brush biopsies (MBB) as a new diagnostic test for peanut allergies. Twenty individuals participated in this study; each participant underwent oral MBB and serum testing for peanut IgE. The authors note that “At 0.12 kU/L, the sensitivity of oral MBB testing was 80% and the specificity was 85%, whereas at 1.0 kU/L, the sensitivity of sIgE testing was 50% and the specificity was 100%. From the ROC curves, the areas under the ROC curve (AUC) for oral MBB and sIgE were 0.91 (p < 0.001) and 0.74 (p = 0.007), respectively. Combination testing further increased both sensitivity and accuracy over oral MBB alone” (He & Reisacher, 2019). These results are promising for oral MBB, although more research needs to be completed.
Clinical Utility and Validity
In 1998, Tschopp et al. (1998) compared three diagnostic tests for atopic diseases. Total serum IgE, Phadiatop, and the SPT were compared for 8329 individuals. Current allergic asthma (CAA) and current allergic rhinitis (CAR) were the conditions studied. The prevalence of CAA was 1.8% and prevalence for CAR was 16.3%. The prevalence of positive tests was 29%, 23%, and 23% for Phadiatop, SPT, and IgE, respectively. The results were as follows: “To diagnose current allergic asthma (CAA) and current allergic rhinitis (CAR), the sensitivity of Phadiatop was significantly higher than that of SPT (72.5% vs 65.4%, 77.1% vs 68.4% respectively) and IgE (72.5% vs 56.9%, 77.1% vs 43.9%, respectively. The sensitivity of SPT was significantly higher (68.4% vs 43.9%) than that of IgE to diagnose CAR. When CAA and CAR were excluded, the SPT specificity was significantly higher than that of Phadiatop (77.8% vs 71.9% and 85.9% vs 80.5%, respectively): when CAR was excluded, SPT was significantly higher than IgE (85.9 vs 81.4%). SPT had significantly the best positive predictive value for CAA (5.2% for SPT vs 4.6% for both IgE and Phadiatop) and CAR (48.7% for SPT vs 43.5% for Phadiatop and 31.6% for IgE). The three markers of atopy had roughly the same negative predictive value (NPV) for CAA, but IgE had a significantly lower NPV for CAR than SPT and Phadiatop (88.1% vs 93.3% and 94.7%, respectively). The diagnostic efficiency of SPT was significantly higher than that of Phadiatop (83.1% vs 79.9% and 77.6 vs 71.9%, respectively) to diagnose CAR and CAA. IgE and SPT had equal efficiency (77.6%), which was significantly higher than that of Phadiatop, to diagnose CAA (71.9%) (Tschopp et al., 1998).” The authors concluded that “SPT have the best positive predictive value and the best efficiency to diagnose respiratory atopic diseases. Furthermore, SPT give information on sensitivity to individual allergens and should therefore be used primarily by clinicians to assess respiratory allergic diseases” (Tschopp et al., 1998).
Usmani and Wilkinson (2007) performed a retrospective analysis of patients who had been prick tested to “establish whether an incomplete diagnosis would have been reached if patch testing had been omitted.” The authors observed that if “investigation of allergic skin disease is undertaken by a non‐dermatologist, it is unlikely that patch testing will be performed.” A total of 330 patients had been prick tested in the time period specified. Sixty-eight patients had positive reactions on prick testing, and 36 of those had positive patch tests. Of the 262 patients who had negative prick tests, 121 had positive patch tests (46.1%) of current relevance to patient history in 92 subjects (35.1%). The authors concluded that “omission of patch testing from the investigation of allergic skin disease, even when contact urticaria may be the sole suspected diagnosis, would result in the frequent missed diagnosis of contact allergy” (Usmani & Wilkinson, 2007).
In 2014, a meta-analysis examined the clinical validity of SPT and IgE measurement for food allergy. Twenty-four studies consisting of 2831 participants were included. The results were as follows: “For cows' milk allergy, the pooled sensitivities were 88% (SPT), and 87% (IgE) and specificities were 68% and 48%. For egg, pooled sensitivities were 92% and 93% and specificities were 58% and 49% for SPT and specific-IgE. For wheat, pooled sensitivities were 73% and 83% and specificities were 73% and 43% for SPT and sIgE. For soy, pooled sensitivities were 55% and 83% and specificities were 68% and 38% for SPT and sIgE. For peanut, pooled sensitivities were 95% and 96%, and specificities were 61% and 59% for SPT and sIgE (Soares-Weiser et al., 2014).”
Klemans et al. (2015) examined the diagnostic accuracy of using sIgE to peanut components to improve sensitivity and specificity of peanut allergen testing. Twenty-two studies were included. The authors found that “sIgE to Ara h 2 [a peanut component] showed the best diagnostic accuracy of all diagnostic tests to diagnose peanut allergy. Compared to the currently used SPT and sIgE to peanut extract, sIgE to Ara h 2 was superior in diagnosing peanut allergy” (Klemans et al., 2015). The authors also found that the worst accuracy was observed to be sIgE to Ara8 and Ara9. The authors concluded that “sIgE to Ara 2 should replace SPT and sIgE to peanut extract in daily clinical practice” (Klemans et al., 2015).
Caglayan Sozmen et al. (2015) examined the diagnostic accuracy of using the patch test to avoid oral food challenge (OFC). They found that in 243 children that underwent OFC to suspected food, clinically relevant food allergies were seen in 40 (65%) children to egg and in 22 (35%) to cow's milk. The sensitivity of SPT for both milk and egg were 92%, specificity 91%, PPV 35%, and NPV 93%. Sensitivity, specificity, PPV, and NPV of atopy patch test for both milk and egg were 21%, 73%, 20%, and 74%, respectively.
Santos et al. (2014) studied the performance of basophil activation tests (BAT) as a diagnostic marker for peanut allergy. Forty-three peanut-allergic children, 36 peanut-sensitized but tolerant children, and 25 non–peanut-sensitized nonallergic children underwent SPT, sIgE, and BAT. The authors found that BAT in peanut-allergic children showed a peanut dose-dependent upregulation of CD63 and CD203c while there was no significant response in the other two cohorts. BAT optimal diagnostic cutoffs showed 97% accuracy, 95% PPV, and 98% NPV. BAT allowed reduction of required oral food challenges (OFCs) by two-thirds. BAT proved particularly useful in cases in which specialists could not accurately diagnose peanut allergy with SPT and sIgE to peanut and to Arah2. Using a 2-step diagnostic approach in which BAT was performed only after equivocal SPT or Arah2-sIgE, BAT had a major effect (97% reduction) on the number of OFCs required.
Santos et al. (2015) also studied the utility of BAT to predict the severity and reactivity to peanut during OFCs. They found that “Of the 124 children submitted to OFCs to peanut, 52 reacted with clinical symptoms that ranged from mild oral symptoms to anaphylaxis. Severe reactions occurred in 41% of cases, and 57% reacted to 0.1 g or less of peanut protein. The ratio of the percentage of CD63(+) basophils after stimulation with peanut and after stimulation with anti-IgE (CD63 peanut/anti-IgE) was independently associated with severity, whereas the basophil allergen threshold sensitivity CD-sens (1/EC₅₀ × 100, where EC₅₀ = half maximal effective concentration) value was independently associated with the threshold of allergic reactions to peanut during OFCs. Patients with CD63 peanut/anti-IgE levels of 1.3 or greater had an increased risk of severe reactions (relative risk, 3.4). Patients with a CD-sens value of 84 or greater had an increased risk of reacting to 0.1 g or less of peanut protein (relative risk, 1.9) (Santos et al., 2015).” The authors concluded that “Basophil reactivity is associated with severity, and basophil sensitivity is associated with the threshold of allergic reactions to peanut. CD63 peanut/anti-IgE and CD-sens values can be used to estimate the severity and threshold of allergic reactions during OFCs” (Santos et al., 2015).
Davila et al. (2015) explored the association between total IgE and severity of asthma. A total of 383 patients were included (129 mild, 82 moderate, and 172 severe). Serum IgE levels were noted to vary “markedly” (147% coefficient of variation). The authors did not find an association between total IgE and forced expiratory volume in 1 second (FEV1) or asthma severity; although, the severe subgroup had a higher percentage of patients with > 400 IU/mL. Independent predictors of higher IgE were found to be younger age, sensitization to ≥ 2 allergens, male gender, and family history of asthma. The authors concluded that “we did not find a significant association between serum total IgE levels and asthma severity or airflow limitation, except for a higher percentage of patients with IgE > 400 IU/mL in the severe subgroup” (Davila et al., 2015).
Tannert et al. (2017) investigated the relevance of a positive skin test and positive IgE test to penicillin allergy. Twenty-five patients with positive results were given penicillin, and another 19 patients deemed allergic were included. However, only 9 of the 25 patients given penicillin were challenge positive. Positive results from each test alone did not predict allergy. The authors concluded that “the best predictor for a clinically significant (IgE-mediated) penicillin allergy is a combination of a positive case history with simultaneous positive ST result and s-IgE or a positive challenge result” (Tannert et al., 2017).
Suárez-Fariñas et al. (2021) investigated the validity of the peanut BBEA diagnostic test on 133 subjects as well as on 82 additional subjects from another study, forming a cohort for a paper titled, “Accurate and reproducible diagnosis of peanut allergy using epitope mapping.” The authors measured levels of IgE to epitopes evaluated against a threshold established prior to the study. The peanut BBEA diagnostic test diagnosed 93% of subjects accurately, with a sensitivity threshold of 92% and specificity of 94%. The positive predictive value (PPV) was 91%. The authors concluded that “the overall accuracy was found to be superior to existing diagnostic tests for peanut allergy including skin prick testing, peanut sIgE, and peanut component sIgE testing” (Suárez-Fariñas et al., 2021).
The American Academy of Allergy, Asthma, and Immunology (AAAAI) and the American College of Allergy, Asthma, and Immunology (ACAAI)
The AAAI and ACAAI published practice parameters in 2008 for allergy testing (Bernstein et al., 2008) which noted that “For individual patients, the choice of test allergens is guided by the history and physical examination and the physician’s knowledge, training, and experience.” The guidelines recommended that “Specific IgE immunoassays may be preferable to skin testing under special clinical conditions, such as widespread skin disease, patients receiving skin test suppressive therapy, uncooperative patients, or when the history suggests an unusually greater risk of anaphylaxis from skin testing.” They also note that for both skin testing and in-vitro specific IgE testing, “the allergens selected … should be determined based on the patient’s age, history, environment and living conditions (e.g., region of the country), occupation, and activities.” Also, “The best indicators in the selection of appropriate pollens for clinical use are extensive prevalence in the air and concurrent allergy symptoms during annually recurrent seasons when such pollens are expected to be present in the ambient air.”
They AAAAI and ACAAI guidelines also state, “As is the case with skin tests, a direct correlation cannot be assumed between the presence of specific IgE (sIgE) antibodies and clinical disease.” Additionally, “sensitivity and the positive predictive value of both prick/puncture and specific IgE tests generally tend to be higher among pollens, stable anaphylactogenic foods, house dust mite, certain epidermals, and fungi compared with venoms, drugs, and chemicals.”
With regards to total IgE testing, these groups indicate, “Measurements of total serum IgE concentration are of modest clinical value when used as a screen for allergic disease or for predicting the risk of allergic disease.”
The AAAAI and ACAAI also note that “IgG and IgG subclass antibody tests for food allergy do not have clinical relevance, are not validated, lack sufficient quality control, and should not be performed.”
Regarding basophil activation assays they state, “Histamine and leukotriene release measurements from human basophils after incubation with allergen are valuable research tools for in vitro investigations of allergy (Bernstein et al., 2008).”
Their practice parameter on drug allergy also states that “The basophil activation test is a recently described method of evaluating expression of CD63 on basophils after stimulation with an allergen. There are limited data using this method to evaluate patients with possible allergies to β-lactam antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs)” (Boyce et al., 2010).
They also recommend, “Because anaphylactic reactions cannot be distinguished from anaphylactoid, nonimmune occurrences, it has been recommended that plasma histamine, tryptase, and specific IgEs (if available) may be ordered at the time of reaction and skin tests be performed later” (Boyce et al., 2010).
In their 2014 practice parameter on food allergy (Sampson et al., 2014) they acknowledge: “Basophil and eosinophilic reactivity tests have been shown to be associated with food-induced allergic responses and have been shown in current research to be modified over time during immunotherapy.”
Their 2014 practice parameter on rhinosinusitis also recommends to “Perform an evaluation for specific IgE antibodies to airborne allergens in patients with RARS or CRS.” An updated practice parameter on rhinitis published in 2020 comments that local allergic rhinitis will often be associated with “negative skin prick tests (and intradermal tests, when performed) and absence of serum-specific IgE (sIgE) antibodies but a positive nasal allergen provocation test (NAPT) to aeroallergens (Dykewicz et al., 2020). With respect to vasomotor rhinitis, the authors state that “laboratory tests, skin prick tests, and sIgE are helpful only to exclude AR [allergic rhinitis].”
In this practice parameter, they also make the following summary concerning re-evaluation of food allergies in children and adolescents: “Summary Statement 11: Consider the natural course of allergies to specific foods when deciding on the frequency of food allergy follow-up evaluations, recognizing that allergies to certain foods (milk, egg, wheat, and soy) generally resolve more quickly in childhood than others (peanut, tree nuts, fish, and shellfish). These observations could support individualized follow-up (ie, roughly yearly re-evaluations of these allergies in childhood) with less frequent retesting if results remain particularly high (e.g., > 20 – 50 kUA/L). [Strength of recommendation: Moderate; C Evidence].”
In their 2015 practice parameter on anaphylaxis (Lieberman et al., 2015), they recommend “Skin tests and/or in vitro tests for specific IgE and challenge tests might be appropriate to help define the cause of the anaphylaxis.”
They also recommend against routinely obtaining total serum IgE levels for the diagnosis of food allergy, however, because of the low PPV of self-reported symptoms and lack of pathognomonic signs on physical examination, they recommend that the accurate diagnosis of IgE-mediated food allergy should be aided by laboratory allergy testing, including skin prick and/or serum IgE testing. The clinician should use specific IgE tests (skin prick tests, serum tests, or both) to foods as diagnostic tools; however, testing should be focused on foods suspected of provoking the reaction, and test results alone should not be considered diagnostic of food allergy. Moreover, “The diagnosis of food-induced anaphylaxis should be based on signs and symptoms in association with likely or known exposure to a food allergen”, as “Events mimicking anaphylaxis also can occur after the ingestion of food” (Lieberman et al., 2015).
In a Choosing Wisely (CW) report, the AAAAI recommends against performing “unproven diagnostic tests, such as immunoglobulin G (lgG) testing or an indiscriminate battery of immunoglobulin E (lgE) tests, in the evaluation of allergy” (AAAAI, 2012).
In another CW report, the AAAAI recommends against routine diagnostic testing in patients with chronic urticaria, stating that “skin or serum-specific IgE testing for inhalants or foods is not indicated, unless there is a clear history implicating an allergen as a provoking or perpetuating factor for urticaria” (AAAAI, 2012).
The AAAAI also published a 2020 practice parameter update on peanut allergy diagnosis. The authors recommend in favor of diagnostic skin prick test or sIgE testing for peanut allergy in patients with physician-judged high pretest probability of peanut allergy. Testing is also recommended prior to an oral food challenge for patients with moderate pretest probability of peanut allergy. Ara h 2 diagnostic testing is the suggested approach for patients presenting for evaluation of suspected peanut allergy for which a single diagnostic test is to be used, due to its superior diagnostic accuracy “by virtue of more optimal positive/negative likelihood ratios.” However, Ara h 2 is noted to have lower sensitivity than the skin prick or sIgE tests, so a clinician may use Ara h 2, SPT, or sIgE to confirm the diagnosis of peanut allergy in a patient with a high prior probability. The AAAAI recommends against “routine use of component testing in addition to either SPT or sIgE to whole peanut to increase diagnostic accuracy”, and also against using the results of skin prick or sIgE to determine “the severity of an allergy phenotype or to predict the severity of a future reaction.”
It is noteworthy that all the the recommendations above were assigned “low” or “very low” degrees of evidence certainty (Greenhawt et al., 2020).
Joint Task Force on Practice Parameters (JTFPP)
In a practice parameter concerning contact dermatitis, the Joint Task Force on Practice Parameters — composed of the American Academy of Allergy, Asthma & Immunology (AAAAI), the American College of Allergy, Asthma & Immunology (ACAAI), and the Joint Council of Allergy, Asthma & Immunology — proposed this series of summary statements:
“Summary Statement 1: Consider ACD [allergic contact dermatitis] in the differential diagnosis of patients with chronic eczematous or noneczematous dermatitis. [Strength of Recommendation: Strong; C Evidence]
Summary Statement 2: In patients suspected of ACD, patch testing is the gold standard to confirm the diagnosis. [Strength of Recommendation: Strong; C Evidence]
Summary Statement 3: In addition to personal products used by a patient suspected of ACD, review the home and workplace for other sources of contact allergens. [Strength of Recommendation: Moderate; D Evidence] Summary Statement
Summary Statement 4: Evaluate patients for both irritant and allergic causes, especially in those presenting with hand dermatitis. [Strength of Recommendation: Strong; C Evidence]
Summary Statement 5: Allergic CD should be suspected and evaluated in the patient with both generalized and anatomically localized skin eruptions (such as the hands, face, eyelids) that come in contact with the substances in the environment. [Strength of Recommendation: Moderate; C Evidence]
Summary Statement 6: In a patient with a facial rash involving the periorbital areas (e.g., eyelids), evaluate for ACD caused by components of cosmetics, such as fragrances, preservatives, and excipients, because these are common sensitizers of the facial skin. [Strength of Recommendation: Moderate; C Evidence]
Summary Statement 7: Evaluate patients presenting with lip dermatitis (cheilitis) and perioral dermatitis for both irritant and allergic causes of contact dermatitis. [Strength of Recommendation: Moderate; C Evidence]
Summary Statement 8: Evaluate patients with chronic oral mucosal inflammatory conditions for disorders other than ACD. [Strength of Recommendation: Moderate; C Evidence]
Summary Statement 9: In patients presenting with dermatitis that involves the scalp and neck, consider patch testing for common causative sensitizers in cosmetics, hair products, and jewelry. [Strength of Recommendation: Moderate; C Evidence]
Summary Statement 10: Consider irritant and ACD in all patients presenting with acute or chronic hand eczema. All such patients suspected of CD should undergo patch testing. [Strength of Recommendation: Moderate; C Evidence]
Summary Statement 11: Evaluate patients with axillary dermatitis for ACD caused by local contact sensitivity to allergens in topically applied products found in deodorants and textiles. In some cases, axillary dermatitis could be a manifestation of systemic contact dermatitis (SCD) (i.e., “the baboon syndrome”). [Strength of Recommendation: Moderate; C Evidence]
Summary Statement 12: Evaluate patients presenting with anogenital dermatitis for possible ACD to antigens contained in topically applied products. [Strength of Recommendation: Moderate; C Evidence]
Summary Statement 13: Consider a diagnosis of SCD following systemic exposure (e.g., ingestion, infusion, or transcutaneous exposure) to a known contact sensitizer in a patient who presents with generalized dermatitis, intertriginous and flexural exanthema (Baboon syndrome), and/or a flare at previous cutaneous sites of exposure [Strength of Recommendation: Moderate; C Evidence].
Summary Statement 14: Consider PT to rubber chemicals, adhesives, and leather components of footwear in patients presenting with unexplained chronic dermatitis involving the lower extremities, feet and/or soles. [Strength of Recommendation: Moderate; C Evidence]
Summary Statement 15: In addition to avoiding irritants in patients with atopic dermatitis (AD), evaluate for ACD, if suspected, as the 2 dermatologic conditions often coexist in the same patient. [Strength of Recommendation: Moderate; C Evidence]” (Fonacier et al., 2015).
Consensus based statements (CBSs) regarding the diagnosis and management of rhinitis from the JTFPP include the following:
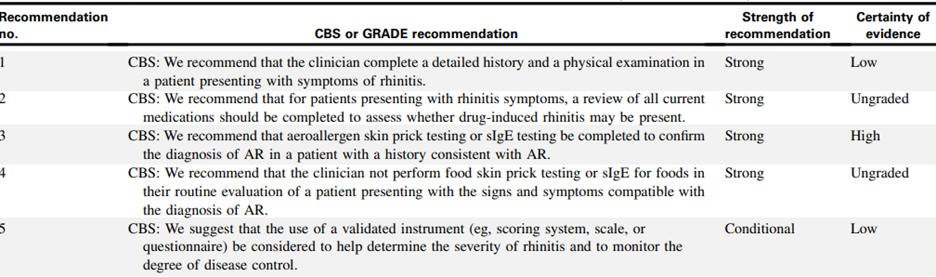
(Dykewicz et al., 2020).
World Allergy Organization Position Paper
In 2020, the World Allergy Organization published a position paper on IgE allergy diagnostics and other relevant allergy tests. Key statements from the paper can be found below:
- “Clinical suspicion of allergic sensitization is confirmed by demonstrating the presence of allergen-specific IgE antibodies in vivo (skin tests) or in vitro.
- Confirmation of allergen sensitization and the identification of causal allergens are essential for optimizing the management of allergic conditions.
- Skin prick testing (SPT) is the most frequently used method for the detection of IgE antibodies, due to its rapidity, simplicity, and low cost. Skin prick tests and other skin test results must be interpreted by a clinician with adequate knowledge of medical history, clinical findings, and relevant type I allergens (including environmental, food, animal, insect, fungal, and drug allergens). Skin tests should include the relevant allergens in the given geographical area and ideally carried out only using standardized allergenic extracts.
- In vitro tests, including molecular based allergy diagnostics, using either in single-plex and in multi-plexed strategies and other more functional tests, such as Basophil Activation Tests allow to better define the IgE profile of the patient. This approach is in line with the Precision Medicine statements” (I. J. Ansotegui et al., 2020)
The paper also states that “Skin tests, especially SPT, represent the most reliable and cost-effective tool for the diagnosis and management of IgE-mediated diseases. They demonstrate a good correlation with outcomes of nasal, conjunctival, dermal, oral and bronchial challenges” (I. J. Ansotegui et al., 2020).
Clinical conditions where SPT is indicated include:
- “Asthma.
- Rhinitis/rhinosinusitis/rhino-conjunctivitis/conjunctivitis.
- Eczema/atopic dermatitis (in the setting of selectively high clinical suspicion for underlying presence of IgE hypersensitivity to specific allergens).
- Suspected food allergy (oral allergy syndrome, anaphylaxis/acute onset or exacerbation of urticaria or eczema that is temporally correlated with food ingestion).
- Suspected drug allergy.
- Hymenoptera venom allergy (systemic reactions immediately following insect sting).
- Suspected occupational disease or exposure to selected potential allergens.
- Chronic urticaria in rare selected cases which strongly suggest an allergen as potential trigger/ aggravating factor.
- Less common disorders, such as eosinophilic esophagitis, eosinophilic gastroenteritis or allergic bronchopulmonary aspergillosis, where IgE sensitization is one of the characteristics of its pathogenesis. However, there is controversy regarding the utility of SPT for these illnesses” (I. J. Ansotegui et al., 2020).
“SPT is not routinely indicated in the following instances in the absence of other existing features of allergic disease:
- Suspected food intolerance (e.g., irritable bowel syndrome, etc.)
- Chronic urticaria in the absence of allergic features in the history
- Desire to lose weight (according to nonconventional approaches, obesity may be due to food intolerance, but no supporting scientific data have been reported in the literature)
- Non-specific food-associated symptoms to food additives/preservatives/colorants
- Evaluation of the effectiveness of allergen immunotherapy (but may be supportive in Hymenoptera venom immunotherapy)
- Non-specific respiratory symptoms to irritants (i.e., smoke, perfumes, detergents, chemicals and other strong odors)
- Screening for allergic sensitization patterns in the absence of clinical symptoms (i.e., family history of allergy)
- Non-specific cutaneous rashes in the absence of atopic features or other allergic symptoms; migraine, except for the indication of specific hypersensitivity to hormones. However, strong scientific data are still missing.
- Chronic fatigue syndrome” (I. J. Ansotegui et al., 2020)
In a 2020 publication on anaphylaxis guidance, the WAO confirms that “allergy testing should be based on patient history and local data regarding the common causes of anaphylaxis in the region. The most frequent elicitor groups worldwide are food, insect venom, and drugs” (Cardona et al., 2020).
World Allergy Organization (WAO), Allergic Rhinitis and its Impact on Asthma (ARIA), and the Global Allergy and Asthma European Network (GA2LEN)
The WAO, ARIA, and GA2LEN published a consensus document in 2020 focused on molecular-based allergy diagnoses. Precision allergy molecular diagnostic applications (PAMD@) “can increase the accuracy of an allergy diagnosis in certain circumstances. In allergic patients, a molecular approach is suitable for the following:
- Assessing the risk of potential allergic reactions, which depend on the individual allergic (clinical) sensitization profile
- Evaluating whether unknown potential triggering factors are present (i.e., the presence of sIgE versus allergenic molecules correlated with high risk for allergic reactions)” (Ignacio J. Ansotegui et al., 2020)
National Institute of Allergy and Infectious Diseases (NIAID)
The NIAID convened an expert panel to review current information and to make recommendations related to the evaluation of food allergy (FA), including the use of specific IgE (sIgE) testing (Boyce et al., 2010). With regards to allergen-specific serum IgE determination, NIAID recommended that “sIgE tests for identifying foods that potentially provoke IgE-mediated food-induced allergic reactions, but alone these tests are not diagnostic of FA.” It stated that “sIgE testing and skin prick testing both depend on the presence of allergen-specific antibodies. Because the former test measures sIgE in the serum and the latter reflects IgE bound to cutaneous mast cells, their results may not always correlate. Serum testing can be especially useful when SPTs cannot be done (for example, due to extensive dermatitis or dermatographism), or when antihistamines cannot be discontinued.” The NIAID also recommended not using the combination of skin prick test (SPT), sIgE tests and atopy patch test (ATP) for the routine diagnosis of food allergy.
Additionally, the NIAID notes that “the routine use of measuring total serum IgE should not be used to make a diagnosis of FA.”
“Non-standardized tests” such as basophil histamine release/activation, lymphocyte stimulation, allergen-specific IgG, cytotoxicity assays, and mediator release assays should not be used in the routine evaluation of FA, according to the NIAID guidelines (Boyce et al., 2010).
In 2017, the NIAID published addendum guidelines for the prevention of peanut allergy in the United States. These guidelines note that the expert panel (EP) “recommends that evaluation with peanut-specific IgE (peanut sIgE) measurement, SPTs, or both be strongly considered before introduction of peanut to determine if peanut should be introduced and, if so, the preferred method of introduction. To minimize a delay in peanut introduction for children who may test negative, testing for peanut sIgE may be the preferred initial approach in certain health care settings, such as family medicine, pediatrics, or dermatology practices, in which skin prick testing is not routine” (Togias et al., 2017). Further, “The EP does not recommend food allergen panel testing or the addition of sIgE testing for foods other than peanut because of their poor positive predictive value, which could lead to misinterpretation, overdiagnosis of food allergy, and unnecessary dietary restrictions” (Togias et al., 2017). More, if an infant has severe eczema, an egg allergy, or both, the EP recommends to “Strongly consider evaluation by sIgE measurement and/or SPT and, if necessary, an OFC. Based on test results, introduce peanut-containing foods” (Togias et al., 2017).
American Academy of Pediatrics (AAP)
In 2012, AAP released a clinical report on allergy testing in childhood. It stated that “Both serum sIgE tests and SPT are sensitive and have similar diagnostic properties.” The AAP summary included the following:
- “Treatment decisions for infants and children with allergy should be made on the basis of history and, when appropriate, identified through directed serum sIgE or SPT testing. Newer in vitro sIgE tests have supplanted radioallergosorbent tests.”
- “Positive sIgE test results indicate sensitization but are not equivalent to clinical allergy. Large panels of indiscriminately performed screening tests may, therefore, provide misleading information.”
- “Increasingly higher levels of sIgE (higher concentrations on serum tests or SPT wheal size) generally correlate with an increased risk of clinical allergy.”
- “Use of a multiallergen serum test can be helpful for screening for atopic disease if there is a clinical suspicion. If positive, allergen-specific testing may be considered.
- “Tests for allergen-specific IgG antibodies are not helpful for diagnosing allergies (AAP, 2012).”
In 2019, the AAP published new guidelines on the prevention of childhood food allergies and other allergic conditions. This article states that “The new recommendations for the prevention of peanut allergy are based largely on the LEAP trial and are endorsed by the AAP.” The AAP endorsed guidelines were published by Togias et al. (2017) and are noted above. They state that the highest-risk infants (those with severe eczema and/or egg allergies) should be introduced to peanuts by 4 – 6 months; further, allergy testing is strongly advised before peanut introduction. SPT and blood testing for peanut-specific IgE (sIgE) are allowable (Greer et al., 2019; Sicherer, 2017).
In 2020, the AAP published a state-of-the-art review of peanut allergy testing advances and controversies. The article states that “current first-line diagnostic tests for peanut allergy have limited specificity, which may be enhanced with emerging tools such as component-resolved diagnostics.” Like the 2019 guideline, they note that first-line best practices for peanut allergy testing include SPT or serum peanut-specific IgE measurement. While both tests are highly sensitive, neither correlate strongly with reaction severity, according to the AAP (Abrams et al., 2020).
U.S. Food and Drug Administration (FDA) on Xolair
The availability of Xolair for treatment of allergic asthma also has implications for allergy testing. According to the package insert, Xolair is indicated for patients 6 years of age and older with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and whose symptoms are inadequately controlled with inhaled corticosteroids … Determine dose (mg) and dosing frequency by serum total IgE level (IU/mL) measured before the start of treatment, and by body weight (kg).” The prescribing information also notes that “Total IgE levels are elevated during treatment and remain elevated for up to one year after the discontinuation of treatment. Therefore, re-testing of IgE levels during Xolair treatment cannot be used as a guide for dose determination” (FDA, 2016).
International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis
The authors reviewed the existing evidence behind various aspects of evaluation and diagnosis of the AR patient, and developed the following recommendations (Wise et al., 2018):
- “History taking is essential in the diagnosis of AR. Physical examination is recommended in the diagnosis of AR, and when combined with patient history, it increases diagnostic accuracy and excludes alternative causes. Making a presumptive diagnosis of AR on history (ideally combined with physical examination) is reasonable and would not delay treatment initiation. Confirmation with diagnostic testing is required for progression to AIT, or desirable with inadequate response to initial treatment.”
- “Skin‐prick testing (SPT) is recommended for evaluation of allergen sensitivities in appropriately selected patients. Regular use of the same SPT device will allow clinicians to familiarize themselves with it and interpretation of results may therefore be more consistent. The use of standardized allergen extracts can further improve consistency of interpretation. Patients can benefit from identification of their specific sensitivities. SPT is a quick and relatively comfortable way to test several antigens with accuracy similar to other available methods of testing.”
- “Total IgE assessment is an option to assess atopic status. However, the evidence does not support a routine use.”
- “Serum sIgE testing may be used in the evaluation of AR. Using standardized allergens and rigorous proficiency testing on the part of laboratories may improve accuracy. Patients can benefit from identification of their specific sensitivities. Further, in some patients who cannot undergo skin testing, sIgE testing is a safe and effective alternative.”
- “The average pooled sensitivity of SPT is 85% which is often slightly higher than that of serum sIgE testing; however, this is not universally true depending on the allergen tested and the characteristics of the patient. Based on accuracy, convenience, cost, and promptness of results, SPT is often chosen as the first line diagnostic instrument to detect sensitivity to aeroallergens. Intradermal testing can be used as a second line test to exclude reactivity if the clinical suspicion is very high. In cases where dermatographism is present and/or patients are unable to wean off medications that affect skin testing, sIgE testing may be a better choice.”
- “BAT is an option for AR diagnosis when first‐line tests are inconclusive or for measuring response to AIT. Basophil sensitivity may be a useful marker for following response to immunotherapy.”
- “Skin testing is not appropriate in all patients. Absolute or relative contraindications to SPT include uncontrolled or severe asthma, severe or unstable cardiovascular disease, concurrent beta‐blocker therapy, and pregnancy. Certain medications and skin conditions may interfere with skin testing.”
- The list of medications that may interfere with skin testing are as follows: H1, H2, or topical antihistamines, anti‐IgE (omalizumab), leukotriene receptor antagonists, tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), benzodiazepines, topical (cutaneous) or systemic corticosteroids, and topical calcineurin inhibitors (i.e. tacrolimus, picrolimus).
The guideline states that because of the “lack of published studies on this topic, an Aggregate Grade of Evidence and evidence based recommendation cannot be provided.”
The National Academies of Science, Engineering and Medicine
The National Academies of Science, Engineering and Medicine convened an expert committee to review the science and management practices of food allergy. Overall, they found that:
- “Currently, no simple diagnostic tests exist for food allergy.”
- “Food allergy evaluation procedures include a medical history and physical examination, and also may include food-specific skin prick test, food-specific serum immunoglobulin E test, diagnostic food elimination diet, and oral food challenge (OFC). Selection of the specific tests needs to be individualized based on the medical history of each patient.”
- “The BAT shows promising preliminary data, the potential utility is recognized and will require additional validation and standardization. “Guidelines suggest not using the BAT clinically on the grounds that it is nonstandardized, but recognize its use as a research tool (NASEM, 2016).”
In 2017, the National Academies of Science, Engineering and Medicine convened an expert committee to examine critical issues related to food allergy. Regarding diagnosis and prognosis, the committee notes that “physicians [should] use evidence-based, standardized procedures as the basis for food allergy diagnosis and avoid nonstandardized and unproven procedures….When food allergy is suspected, the patient should be evaluated by a physician who has the training and experience to select and interpret appropriate diagnostic tests” (Sicherer et al., 2017).
American Academy of Family Physicians (AAFP)
AAFP’s recommendations for practice state: “Allergy and immunologic testing can help clarify the diagnosis and guide treatment. Immediate immunoglobulin E (IgE) and delayed T cell–mediated reactions are the main types of allergic responses. The allergens suspected in an immediate IgE-mediated response are identified through serum IgE-specific antibody or skin testing. For patients with an inhalant allergy, skin or IgE-specific antibody testing is preferred. In patients with food allergies, eliminating the suspected allergenic food from the diet is the initial treatment. If this is ineffective, IgE-specific antibody or skin testing can exclude allergens. An oral food challenge should be performed to confirm the diagnosis. Patients with an anaphylactic reaction to an insect sting should undergo IgE-specific antibody or skin testing. Skin testing for penicillin has a high negative predictive value and can help when penicillin administration is indicated and there are limited alternatives. Testing for other drug allergies has less well-determined sensitivity and specificity but can guide the diagnosis. Patch testing can help identify the allergen responsible for contact dermatitis (K. L. Chang & J. C. Guarderas, 2018).”
European Academy of Allergy and Clinical Immunology (EAACI)
The EAACI published guidelines on “Biomarkers for monitoring the clinical efficacy of allergen Immunotherapy (AIT).” In it, they concluded that “to date, there are no validated and generally accepted candidate biomarkers that are predictive or indicative of the clinical response to AIT.” However, they did note sIgE/tIgE ratio and IgE‐FAB as candidate biomarkers for future research (Shamji et al., 2017).
The EAACI released a position statement on the BAT. In it, they concluded that “Basophil activation test has been established as a routine diagnostic test with standardized allergen preparations in a number of service laboratories … An important next step is the standardization and automation of analysis of BAT. Once that is achieved, it will be possible to do large multicenter trials to characterize the diagnostic performance of BAT and broaden its use as a clinical tool” (Hoffmann et al., 2015).
National Institute for Health and Care Excellence (NICE)
NICE published a guideline on asthma, recommending against use of serum total or specific IgE for diagnosing asthma. Specific IgE or prick tests to aeroallergens should be used to identify triggers to asthma after a formal diagnosis has been made (NICE, 2021).
NICE also released a statement on multiplex allergen testing, particularly “ImmunoCAP ISAC” Although they acknowledge the test’s promise, they state that there is “insufficient evidence to recommend the routine adoption of multiplex allergen testing with ImmunoCAP ISAC 112 to help diagnose allergy and predict the risk of an allergic reaction in people with allergy that is difficult to diagnose, when used with standard clinical assessment” (NICE, 2020).
Regarding the assessment and diagnosis of food allergy in under 19s, NICE published the below recommendations:
For food allergies classified as IgE-mediated:
“Based on the results of the allergy-focused clinical history, if IgE-mediated allergy is suspected, offer the child or young person a skin prick test and/or blood tests for specific IgE antibodies to the suspected foods and likely co-allergens.”
“Tests should only be undertaken by healthcare professionals with the appropriate competencies to select, perform and interpret them.”
“Skin prick tests should only be undertaken where there are facilities to deal with an anaphylactic reaction.”
“Choose between a skin prick test and a specific IgE antibody blood test based on:
- The results of the allergy-focused clinical history.
- Whether the test is suitable for, safe for and acceptable to the child or young person (or their parent or carer).
- The available competencies of the healthcare professional to undertake the test and interpret the results.”
“Do not carry out allergy testing without first taking an allergy-focused clinical history. Interpret the results of tests in the context of information from the allergy-focused clinical history.”
“Do not use atopy patch testing or oral food challenges to diagnose IgE-mediated food allergy in primary care or community settings” (NICE, 2011).
References:
- AAAAI. (2012). http://www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/
- AAP. (2012). Allergy Testing in Childhood: Using Allergen-Specific IgE Tests. https://pdfs.semanticscholar.org/51a4/dfa0a84e4dc8b0893529a6197bd9b94bdbfe.pdf
- Abrams, E. M., Chan, E. S., & Sicherer, S. (2020). Peanut Allergy: New Advances and Ongoing Controversies. Pediatrics, 145(5), e20192102. https://doi.org/10.1542/peds.2019-2102
- Adams, P. F., Kirzinger, W. K., & Martinez, M. (2013). Summary health statistics for the U.S. population: National Health Interview Survey, 2012. Vital Health Stat 10(259), 1-95. https://www.cdc.gov/nchs/data/series/sr_10/sr10_259.pdf
- Ansotegui, I. J., Melioli, G., Canonica, G. W., Caraballo, L., Villa, E., Ebisawa, M., Passalacqua, G., Savi, E., Ebo, D., Gómez, R. M., Luengo Sánchez, O., Oppenheimer, J. J., Jensen-Jarolim, E., Fischer, D. A., Haahtela, T., Antila, M., Bousquet, J. J., Cardona, V., Chiang, W. C., . . . Zuberbier, T. (2020). IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J, 13(2), 100080. https://doi.org/10.1016/j.waojou.2019.100080
- Ansotegui, I. J., Melioli, G., Canonica, G. W., Gomez, R. M., Jensen-Jarolim, E., Ebisawa, M., Luengo, O., Caraballo, L., Passalacqua, G., Poulsen, L., Savi, E., Zuberbier, T., Villa, E., & Oppenheimer, J. (2020). A WAO - ARIA - GA(2)LEN consensus document on molecular-based allergy diagnosis (PAMD@): Update 2020. World Allergy Organ J, 13(2), 100091. https://doi.org/10.1016/j.waojou.2019.100091
- Bahri, R., Custovic, A., Korosec, P., Tsoumani, M., Barron, M., Wu, J., Sayers, R., Weimann, A., Ruiz-Garcia, M., Patel, N., Robb, A., Shamji, M. H., Fontanella, S., Silar, M., Mills, E., Simpson, A., Turner, P. J., & Bulfone-Paus, S. (2018). Mast cell activation test in the diagnosis of allergic disease and anaphylaxis. In J Allergy Clin Immunol (Vol. 142, pp. 485-496 e416). https://doi.org/10.1016/j.jaci.2018.01.043
- Bernstein, I. L., Li, J. T., Bernstein, D. I., Hamilton, R., Spector, S. L., Tan, R., Sicherer, S., Golden, D. B., Khan, D. A., Nicklas, R. A., Portnoy, J. M., Blessing-Moore, J., Cox, L., Lang, D. M., Oppenheimer, J., Randolph, C. C., Schuller, D. E., Tilles, S. A., Wallace, D. V., . . . Weber, R. (2008). Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol, 100(3 Suppl 3), S1-148. https://doi.org/https://doi.org/10.1016/S1081-1206(10)60305-5
- Beyer, K., & Teuber, S. S. (2005). Food allergy diagnostics: scientific and unproven procedures. Curr Opin Allergy Clin Immunol, 5(3), 261-266. https://journals.lww.com/co-allergy/Abstract/2005/06000/Food_allergy_diagnostics__scientific_and_unproven.12.aspx
- BioSpace. (2021). AllerGenis Allergy Diagnostic Company to Present Significant Milestones at the Biotech Showcase(TM) 2021. https://www.biospace.com/article/allergenis-allergy-diagnostic-company-to-present-significant-milestones-at-the-biotech-showcase-tm-2021/
- Boyce, J. A., Assa'ad, A., Burks, A. W., Jones, S. M., Sampson, H. A., Wood, R. A., Plaut, M., Cooper, S. F., Fenton, M. J., Arshad, S. H., Bahna, S. L., Beck, L. A., Byrd-Bredbenner, C., Camargo, C. A., Jr., Eichenfield, L., Furuta, G. T., Hanifin, J. M., Jones, C., Kraft, M., . . . Schwaninger, J. M. (2010). Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol, 126(6), 1105-1118. https://doi.org/10.1016/j.jaci.2010.10.008
- Caglayan Sozmen, S., Povesi Dascola, C., Gioia, E., Mastrorilli, C., Rizzuti, L., & Caffarelli, C. (2015). Diagnostic accuracy of patch test in children with food allergy. Pediatr Allergy Immunol, 26(5), 416-422. https://doi.org/10.1111/pai.12377
- Cardona, V., Ansotegui, I. J., Ebisawa, M., El-Gamal, Y., Fernandez Rivas, M., Fineman, S., Geller, M., Gonzalez-Estrada, A., Greenberger, P. A., Sanchez Borges, M., Senna, G., Sheikh, A., Tanno, L. K., Thong, B. Y., Turner, P. J., & Worm, M. (2020). World allergy organization anaphylaxis guidance 2020. World Allergy Organ J, 13(10), 100472. https://doi.org/10.1016/j.waojou.2020.100472
- Carlsson, M., Thorell, L., Sjolander, A., & Larsson-Faria, S. (2015). Variability of total and free IgE levels and IgE receptor expression in allergic subjects in and out of pollen season. Scand J Immunol, 81(4), 240-248. https://doi.org/10.1111/sji.12270
- CellScienceSystems. (2021). Identify food and chemical sensitivities with the Alcat Test. https://cellsciencesystems.com/providers/alcat-test/
- Chang, K.-L., & Guarderas, J. C. (2018). Allergy Testing: Common Questions and Answers. American Family Physician, 98(1), 34-39. /afp/2018/0701/p34.pdf
- Chang, K. L., & Guarderas, J. C. (2018). Allergy Testing: Common Questions and Answers. Am Fam Physician, 98(1), 34-39. https://www.aafp.org/pubs/afp/issues/2018/0701/p34.html
- Chow, A. W., Benninger, M. S., Brook, I., Brozek, J. L., Goldstein, E. J., Hicks, L. A., Pankey, G. A., Seleznick, M., Volturo, G., Wald, E. R., & File, T. M., Jr. (2012). IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis, 54(8), e72-e112. https://doi.org/10.1093/cid/cir1043
- Davila, I., Valero, A., Entrenas, L. M., Valveny, N., & Herraez, L. (2015). Relationship between serum total IgE and disease severity in patients with allergic asthma in Spain. J Investig Allergol Clin Immunol, 25(2), 120-127. http://www.jiaci.org/issues/vol25issue2/vol25issue02-5.htm
- Depince-Berger, A. E., Sidi-Yahya, K., Jeraiby, M., & Lambert, C. (2017). Basophil activation test: Implementation and standardization between systems and between instruments. Cytometry A, 91(3), 261-269. https://doi.org/10.1002/cyto.a.23078
- Dykewicz, M. S., Wallace, D. V., Amrol, D. J., Baroody, F. M., Bernstein, J. A., Craig, T. J., Dinakar, C., Ellis, A. K., Finegold, I., Golden, D. B. K., Greenhawt, M. J., Hagan, J. B., Horner, C. C., Khan, D. A., Lang, D. M., Larenas-Linnemann, D. E. S., Lieberman, J. A., Meltzer, E. O., Oppenheimer, J. J., . . . Steven, G. C. (2020). Rhinitis 2020: A practice parameter update. J Allergy Clin Immunol, 146(4), 721-767. https://doi.org/10.1016/j.jaci.2020.07.007
- FDA. (2016). Xolair Label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/103976s5225lbl.pdf
- Fonacier, L., Bernstein, D. I., Pacheco, K., Holness, D. L., Blessing-Moore, J., Khan, D., Lang, D., Nicklas, R., Oppenheimer, J., Portnoy, J., Randolph, C., Schuller, D., Spector, S., Tilles, S., & Wallace, D. (2015). Contact dermatitis: a practice parameter-update 2015. J Allergy Clin Immunol Pract, 3(3 Suppl), S1-39. https://doi.org/10.1016/j.jaip.2015.02.009
- Genova. (2021). Allergix® IgG4 Food Antibodies 90 - Serum. https://www.gdx.net/product/allergix-igg4-food-antibodies-90-food-sensitivity-test-serum
- Greenhawt, M., Shaker, M., Wang, J., Oppenheimer, J. J., Sicherer, S., Keet, C., Swaggart, K., Rank, M., Portnoy, J. M., Bernstein, J., Chu, D. K., Dinakar, C., Golden, D., Horner, C., Lang, D. M., Lang, E. S., Khan, D. A., Lieberman, J., Stukus, D., & Wallace, D. (2020). Peanut allergy diagnosis: A 2020 practice parameter update, systematic review, and GRADE analysis. J Allergy Clin Immunol, 146(6), 1302-1334. https://doi.org/10.1016/j.jaci.2020.07.031
- Greer, F. R., Sicherer, S. H., & Burks, A. W. (2019). The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics, 143(4). https://doi.org/10.1542/peds.2019-0281
- Hamilton, R. (2021). Allergen sampling in the environment - UpToDate (P. S. Creticos & A. Feldweg, Eds.) https://www.uptodate.com/contents/allergen-sampling-in-the-environment
- Hamilton, R. G., Matsson, P. N., Hovanec-Burns, D. L., Van Cleve, M., Chan, S., Kober, A., Kleine-Tebbe, J. R., Renz, H., Magnusson, C., & Quicho, R. (2015). Analytical Performance Characteristics, Quality Assurance and Clinical Utility of Immunological Assays for Human IgE Antibodies of Defined Allergen Specificities.(CLSI-ILA20-A3). Journal of Allergy and Clinical Immunology, 135(2), AB8. https://www.jacionline.org/article/S0091-6749(14)02742-0/fulltext
- Hammond, C., & Lieberman, J. A. (2018). Unproven Diagnostic Tests for Food Allergy. Immunol Allergy Clin North Am, 38(1), 153-163. https://doi.org/10.1016/j.iac.2017.09.011
- He, Y. T., & Reisacher, W. R. (2019). Sensitivity, specificity, and predictive value of oral mucosal brush biopsy for the diagnosis of peanut allergy. Int Forum Allergy Rhinol, 9(6), 624-628. https://doi.org/10.1002/alr.22302
- Hemmings, O., Kwok, M., McKendry, R., & Santos, A. F. (2018). Basophil Activation Test: Old and New Applications in Allergy. Current Allergy and Asthma Reports, 18(12), 77. https://doi.org/10.1007/s11882-018-0831-5
- Hoffmann, H. J., Santos, A. F., Mayorga, C., Nopp, A., Eberlein, B., Ferrer, M., Rouzaire, P., Ebo, D. G., Sabato, V., Sanz, M. L., Pecaric-Petkovic, T., Patil, S. U., Hausmann, O. V., Shreffler, W. G., Korosec, P., & Knol, E. F. (2015). The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy, 70(11), 1393-1405. https://doi.org/10.1111/all.12698
- Jackson, K. D., Howie., L. D., Akinbami, L. J., & CDC. (2013). Trends in Allergic Conditions Among Children: United States, 1997-2011. NCHS Data Brief. No 121. https://www.cdc.gov/nchs/products/databriefs/db121.htm
- Kim, S. Y., Kim, J. H., Jang, Y. S., Choi, J. H., Park, S., Hwang, Y. I., Jang, S. H., & Jung, K. S. (2016). The Basophil Activation Test Is Safe and Useful for Confirming Drug-Induced Anaphylaxis. Allergy Asthma Immunol Res, 8(6), 541-544. https://doi.org/10.4168/aair.2016.8.6.541
- Klemans, R. J., van Os-Medendorp, H., Blankestijn, M., Bruijnzeel-Koomen, C. A., Knol, E. F., & Knulst, A. C. (2015). Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: a systematic review. Clin Exp Allergy, 45(4), 720-730. https://doi.org/10.1111/cea.12412
- Knight, V., Wolf, M. L., Trikha, A., Curran-Everett, D., Hiserote, M., & Harbeck, R. J. (2018). A comparison of specific IgE and skin prick test results to common environmental allergens using the HYTEC™ 288. Journal of Immunological Methods, 462, 9-12. https://doi.org/https://doi.org/10.1016/j.jim.2018.07.005
- Kowal, K., & DuBuske, L. (2021, 05/03/2021). Overview of in vitro allergy tests. https://www.uptodate.com/contents/overview-of-in-vitro-allergy-tests
- Kowal, K., & DuBuske, L. (2022, 11/30/2022). Overview of skin testing for allergic disease - UpToDate. https://www.uptodate.com/contents/overview-of-skin-testing-for-allergic-disease
- Lieberman, P., Nicklas, R. A., Randolph, C., Oppenheimer, J., Bernstein, D., Bernstein, J., Ellis, A., Golden, D. B., Greenberger, P., Kemp, S., Khan, D., Ledford, D., Lieberman, J., Metcalfe, D., Nowak-Wegrzyn, A., Sicherer, S., Wallace, D., Blessing-Moore, J., Lang, D., . . . Tilles, S. A. (2015). Anaphylaxis--a practice parameter update 2015. Ann Allergy Asthma Immunol, 115(5), 341-384. https://doi.org/10.1016/j.anai.2015.07.019
- Mowad, C. M. (2006). Patch testing: pitfalls and performance. Curr Opin Allergy Clin Immunol, 6(5), 340-344. https://doi.org/10.1097/01.all.0000244794.03239.8e
- NASEM. (2016). Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management, and Public Policy (Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management, and Public Policy, Issue. http://dx.doi.org/10.17226/23658
- Nelson, H. S. (2001). Variables in Allergy Skin Testing. Immunology and Allergy Clinics, 21(2), 281-290. https://doi.org/10.1016/S0889-8561(05)70206-X
- NICE. (2011, February 23, 2011). Food allergy in under 19s: assessment and diagnosis. National Institute for Health and Care Excellence (NICE). https://www.nice.org.uk/guidance/cg116/chapter/Recommendations#ige-mediated-food-allergy
- NICE. (2020). ImmunoCAP ISAC 112 for multiplex allergen testing. https://www.nice.org.uk/guidance/dg24/chapter/1-Recommendations
- NICE. (2021). Asthma: diagnosis, monitoring and chronic asthma management. https://www.nice.org.uk/guidance/ng80
- Oppenheimer, J., & Nelson, H. S. (2006). Skin testing: a survey of allergists. Ann Allergy Asthma Immunol, 96(1), 19-23. https://doi.org/10.1016/s1081-1206(10)61034-4
- Pawankar, R., Holgate, S. T., Canonica, G. W., Lockey, R. F., & Blaiss, M. S. (2013). WAO White Book on Allergy | World Allergy Organization. http://www.worldallergy.org/wao-white-book-on-allergy
- Rietschel, R. L. (1997). COMPARISON OF ALLERGIC AND IRRITANT CONTACT DERMATITIS. Immunology and Allergy Clinics, 17(3), 359-364. https://doi.org/10.1016/S0889-8561(05)70314-3
- Sampson, H. A., Aceves, S., Bock, S. A., James, J., Jones, S., Lang, D., Nadeau, K., Nowak-Wegrzyn, A., Oppenheimer, J., Perry, T. T., Randolph, C., Sicherer, S. H., Simon, R. A., Vickery, B. P., Wood, R., Bernstein, D., Blessing-Moore, J., Khan, D., Nicklas, R., . . . Wallace, D. (2014). Food allergy: a practice parameter update-2014. J Allergy Clin Immunol, 134(5), 1016-1025.e1043. https://doi.org/10.1016/j.jaci.2014.05.013
- Santos, A. F., Douiri, A., Becares, N., Wu, S. Y., Stephens, A., Radulovic, S., Chan, S. M., Fox, A. T., Du Toit, G., Turcanu, V., & Lack, G. (2014). Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J Allergy Clin Immunol, 134(3), 645-652. https://doi.org/10.1016/j.jaci.2014.04.039
- Santos, A. F., Du Toit, G., Douiri, A., Radulovic, S., Stephens, A., Turcanu, V., & Lack, G. (2015). Distinct parameters of the basophil activation test reflect the severity and threshold of allergic reactions to peanut. J Allergy Clin Immunol, 135(1), 179-186. https://doi.org/10.1016/j.jaci.2014.09.001
- Santos, A. F., & Lack, G. (2016). Basophil activation test: food challenge in a test tube or specialist research tool? Clin Transl Allergy, 6, 10. https://doi.org/10.1186/s13601-016-0098-7
- Santos, A. F., & Shreffler, W. G. (2017). Road map for the clinical application of the basophil activation test in food allergy. Clin Exp Allergy, 47(9), 1115-1124. https://doi.org/10.1111/cea.12964
- Shamji, M. H., Kappen, J. H., Akdis, M., Jensen-Jarolim, E., Knol, E. F., Kleine-Tebbe, J., Bohle, B., Chaker, A. M., Till, S. J., Valenta, R., Poulsen, L. K., Calderon, M. A., Demoly, P., Pfaar, O., Jacobsen, L., Durham, S. R., & Schmidt-Weber, C. B. (2017). Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy, 72(8), 1156-1173. https://doi.org/10.1111/all.13138
- Sicherer, S. (2017). New guidelines detail use of ‘infant-safe’ peanut to prevent allergy. https://www.aappublications.org/news/2017/01/05/PeanutAllergy010517
- Sicherer, S. H., Allen, K., Lack, G., Taylor, S. L., Donovan, S. M., & Oria, M. (2017). Critical Issues in Food Allergy: A National Academies Consensus Report. Pediatrics, 140(2). https://doi.org/10.1542/peds.2017-0194
- Siroux, V., Lupinek, C., Resch, Y., Curin, M., Just, J., Keil, T., Kiss, R., Lodrup Carlsen, K., Melen, E., Nadif, R., Pin, I., Skrindo, I., Vrtala, S., Wickman, M., Anto, J. M., Valenta, R., & Bousquet, J. (2017). Specific IgE and IgG measured by the MeDALL allergen-chip depend on allergen and route of exposure: The EGEA study. J Allergy Clin Immunol, 139(2), 643-654.e646. https://doi.org/10.1016/j.jaci.2016.05.023
- Soares-Weiser, K., Takwoingi, Y., Panesar, S. S., Muraro, A., Werfel, T., Hoffmann-Sommergruber, K., Roberts, G., Halken, S., Poulsen, L., van Ree, R., Vlieg-Boerstra, B. J., & Sheikh, A. (2014). The diagnosis of food allergy: a systematic review and meta-analysis. Allergy, 69(1), 76-86. https://doi.org/10.1111/all.12333
- Sookrung, N., Jotikaprasardhna, P., Bunnag, C., Chaicumpa, W., & Tungtrongchitr, A. (2019). Concordance of skin prick test and serum-specific IgE to locally produced component-resolved diagnostics for cockroach allergy. Ann Allergy Asthma Immunol, 122(1), 93-98. https://doi.org/10.1016/j.anai.2018.09.463
- Spiriplex. (2021). Allergies. https://spiriplex.com/allergies/
- Stokes, J., & Casale, T. (2022, 11/22/2022). The relationship between IgE and allergic disease. https://www.uptodate.com/contents/the-relationship-between-ige-and-allergic-disease
- Suárez-Fariñas, M., Suprun, M., Kearney, P., Getts, R., Grishina, G., Hayward, C., Luta, D., Porter, A., Witmer, M., du Toit, G., Lack, G., Chinthrajah, R. S., Galli, S. J., Nadeau, K., & Sampson, H. A. (2021). Accurate and reproducible diagnosis of peanut allergy using epitope mapping. Allergy, n/a(n/a). https://doi.org/https://doi.org/10.1111/all.14905
- Tannert, L. K., Mortz, C. G., Skov, P. S., & Bindslev-Jensen, C. (2017). Positive Skin Test or Specific IgE to Penicillin Does Not Reliably Predict Penicillin Allergy. J Allergy Clin Immunol Pract, 5(3), 676-683. https://doi.org/10.1016/j.jaip.2017.03.014
- Togias, A., Cooper, S. F., Acebal, M. L., Assa'ad, A., Baker, J. R., Jr., Beck, L. A., Block, J., Byrd-Bredbenner, C., Chan, E. S., Eichenfield, L. F., Fleischer, D. M., Fuchs, G. J., 3rd, Furuta, G. T., Greenhawt, M. J., Gupta, R. S., Habich, M., Jones, S. M., Keaton, K., Muraro, A., . . . Boyce, J. A. (2017). Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol, 139(1), 29-44. https://doi.org/10.1016/j.jaci.2016.10.010
- Tschopp, J. M., Sistek, D., Schindler, C., Leuenberger, P., Perruchoud, A. P., Wuthrich, B., Brutsche, M., Zellweger, J. P., Karrer, W., & Brandli, O. (1998). Current allergic asthma and rhinitis: diagnostic efficiency of three commonly used atopic markers (IgE, skin prick tests, and Phadiatop). Results from 8329 randomized adults from the SAPALDIA Study. Swiss Study on Air Pollution and Lung Diseases in Adults. Allergy, 53(6), 608-613. https://pubmed.ncbi.nlm.nih.gov/9689343/
- Usmani, N., & Wilkinson, S. M. (2007). Allergic skin disease: investigation of both immediate- and delayed-type hypersensitivity is essential. Clin Exp Allergy, 37(10), 1541-1546. https://doi.org/10.1111/j.1365-2222.2007.02805.x
- Werther, R. L., Choo, S., Lee, K. J., Poole, D., Allen, K. J., & Tang, M. L. (2012). Variability in Skin Prick Test Results Performed by Multiple Operators Depends on the Device Used. World Allergy Organ J, 5(12), 200-204. https://doi.org/10.1097/WOX.0b013e31827e6513
- Wise, S. K., Lin, S. Y., Toskala, E., Orlandi, R. R., Akdis, C. A., Alt, J. A., Azar, A., Baroody, F. M., Bachert, C., Canonica, G. W., Chacko, T., Cingi, C., Ciprandi, G., Corey, J., Cox, L. S., Creticos, P. S., Custovic, A., Damask, C., DeConde, A., . . . Zacharek, M. (2018). International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. Int Forum Allergy Rhinol, 8(2), 108-352. https://doi.org/10.1002/alr.22073
- Wuthrich, B. (2005). Unproven techniques in allergy diagnosis. J Investig Allergol Clin Immunol, 15(2), 86-90. https://pubmed.ncbi.nlm.nih.gov/16047707/
- Zug, K. A., Pham, A. K., Belsito, D. V., DeKoven, J. G., DeLeo, V. A., Fowler, J. F., Jr., Fransway, A. F., Maibach, H. I., Marks, J. G., Jr., Mathias, C. G., Pratt, M. D., Sasseville, D., Storrs, F. J., Taylor, J. S., Warshaw, E. M., & Zirwas, M. J. (2014). Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis, 25(6), 345-355. https://doi.org/10.1097/der.0000000000000083
Coding Section
|
Code |
Number |
Code Description |
|
CPT |
82784 |
Gammaglobulin (immunoglobulin); IgA, IgD, IgG, IgM, each |
|
|
82785 |
Gammaglobulin; IgE |
|
|
82787 |
Immunoglobulin subclasses (e.g.: IgG 1, 2, 3, or 4) each |
|
|
83516 |
Immunoassay for analyte other than infectious agent antibody or infectious agent antigen; qualitative or semiquantitative, multiple step method (ALCAT) |
|
|
86001 |
Allergen specific IgG quantitative or semi-quantitative, each allergen |
|
|
86003 |
Allergen specific IgE quantitative or semi-quantitative, each allergen |
|
|
86005 |
Qualitative, multi-allergen screen (dipstick, paddle or disk) |
|
|
86008 |
Allergen specific IgE; quantitative or semiquantitative, recombinant or purified component, each |
|
|
88184 |
Flow cytometry, cell surface, cytoplasmic, or nuclear marker, technical component only; first marker [anti-IgE receptor antibody testing](BAT) |
|
|
88185 |
Flow cytometry, cell surface, cytoplasmic, or nuclear marker, technical component only; each additional marker |
|
|
0165U |
Peanut allergen-specific quantitative assessment of multiple epitopes using enzyme-linked immunosorbent assay (ELISA), blood, individual epitope results and probability of peanut allergy |
|
|
0178U |
Peanut allergen-specific quantitative assessment of multiple epitopes using enzyme-linked immunosorbent assay (ELISA), blood, report of minimum eliciting exposure for a clinical reaction |
|
ICD-10 Codes |
F78.A1 |
SYNGAP1-related intellectual disability |
|
|
F78.A9 |
Other genetic related intellectual disability |
|
|
R05.1 |
Acute cough |
|
|
R05.2 |
Subacute cough |
|
|
R05.3 |
Chronic cough |
|
|
R05.4 |
Cough syncope |
|
|
R05.8 |
Other specified cough |
|
|
R05.9 |
Cough, unspecified |
|
|
Z91.010 |
Allergy to peanuts |
|
|
Z91.011. |
Allergy to milk products |
|
|
Z91.012 |
Allergy to eggs |
|
|
Z91.013 |
Allergy to seafood |
|
|
Z91.018 |
Allergy to other foods |
|
|
Z91.02 |
Food additives allergy status |
|
|
Z91.030 |
Bee allergy status |
|
|
Z91.038 |
Other insect allergy status |
|
|
Z91.040 |
Latex allergy status |
|
|
Z91.041 |
Radiographic dye allergy status |
|
|
Z91.048 |
Other nonmedicinal substance allergy status |
|
|
Z91.09 |
Other allergy status, other than to drugs and biological substances |
|
|
Z91.14 |
Patient's other noncompliance with medication regimen |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, Blue Cross Blue Shield Association technology assessment program (TEC) and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2013 Forward
| 04/18/2023 | Interim review, annual review now moved to April. NO change to policy intent, but, policy is being rewritten for clarity and consistency. Also updating description, table of terminology, rationale and references. |
| 01/05/2023 | Annual review, no change to policy intent. |
|
04/27/2022 |
Interim review. Adding coverage criteria #4 and updating criteria 1a. Also updating rationale and references. |
|
10/01/2021 |
Annual review, adding criteria #9 related to non-specific IgE testing. Also updating background, rationale, references and regulatory status. |
|
10/01/2020 |
Annual update, Updating rationale, references, description, policy and coding to include verbiage related to peanut allergy testing. |
|
01/10/2020 |
Interim review, updating criteria for RAST, MAST, FAST, ELISA and ImmunoCAP testing. NO other changes made. |
|
10/23/2019 |
Annual review, Updating Title, description, policy, rationale, regulatory status, references and coding. No other changes made.. |
|
10/09/2019 |
Annual review, no change to policy intent. |
|
11/27/2018 |
Interim review adding investigational verbiage for basophil activation flow cytometry. No other changes. |
|
10/01/2018 |
Annual review, no change to policy intent. |
|
12/7/2017 |
Updating policy with 2018 coding. No other changes. |
|
10/30/2017 |
Interim review adding the following policy verbiage:. No other changes made. |
|
10/02/2017 |
Annual review, no change to policy intent. Updated coding with 2018 codes. |
|
06/19/2017 |
Updated coding section. No other changes. |
|
04/25/2017 |
Updated category to Laboratory. No other changes. |
|
11/07/2016 |
Interim review to place correct number of allergen specific antibodies from 36 to 20. |
|
09/21/2016 |
Annual review, Updating to add coding and verbiage to policy that states" In-vitro specific IgE testing is limited to 20 allergen specific antibodies per year. Additional testing beyond this number will require individual review for coverage criteria. |
|
10/07/2014 |
Annual review, no changes made. |
|
10/30/2013 |
Added Related Policy Section. |