Cardiovascular Disease Risk Assessment - CAM 188
escription
Cardiovascular risk assessment comprises the means and processes to predict the probability of developing a cardiovascular disease. These are a group of tests and health factors that have been proven to indicate a person's chance of having a cardiovascular event such as a heart attack or stroke.
Tests typically used to assess cardiovascular risk include lipid profiles or panels, biomarkers, and cardiovascular risk panels.
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
Coverage Criteria 1, regarding lipid panels, is only intended for adult (18 years or older) patients.
- Lipid Panel
- Measurement of total cholesterol, HDL-C, LDL-C, and triglycerides as part of an assessment of cardiovascular risk factors is considered MEDICALLY NECESSARY:
- Every 4 to 6 years in patients ages 18 to 79 years.
- Annually for patients at increased risk for cardiovascular disease as defined by 2013 ACC/AHA Pooled Cohort Equations (PCEs) to calculate 10-year risk of CVD events (see Note 1).
- A lipid panel is considered MEDICALLY NECESSARY when evaluating an individual at increased risk of dyslipidemia due to the following conditions:
- Nephrotic Syndrome
- Hypothyroidism
- Hyperthyroidism
- Pancreatitis
- Diabetes
- Chronic Kidney Disease
- Cushing Syndrome
- Pregnancy
- Cholestatic Liver Disease
- Lipid metabolism disorders, such as Gaucher disease in adults
- Before beginning statin therapy, a lipid panel is considered MEDICALLY NECESSARY for establishing baseline levels for monitoring therapy.
- For individuals receiving statin therapy, lipid panel testing is considered MEDICALLY NECESSARY up to every four to twelve weeks after initiation or change of therapy. Subsequently, annual lipid panel testing is considered medically necessary for individuals receiving statin therapy.
- Lipid panel testing is considered MEDICALLY NECESSARY for individuals on a long-term drug therapy that requires lipid monitoring, including but not limited to, Accutane and anti-psychotics.
- A lipid panel is considered MEDICALLY NECESSARY when evaluating and managing an individual diagnosed with HIV and receiving antiretroviral therapy (ART):
- Prior to initiating ART (baseline)
- Within one to three months after starting or modifying ART and every 6 to 12 months thereafter
- Measurement of total cholesterol, HDL-C, LDL-C, and triglycerides as part of an assessment of cardiovascular risk factors is considered MEDICALLY NECESSARY:
- Measurement of apolipoprotein B (apoB) is considered MEDICALLY NECESSARY for individuals with one of the following:
- Hypertriglyceridemia
- Diabetes mellitus
- Obesity or metabolic syndrome
- Other dyslipidemias (such as very low LDL-C)
- On lipid therapy
- To facilitate diagnosis of Familial Dysbetalipoproteinemia and Familial Combined Hyperlipidemia
- Measurement of lipoprotein a (Lp(a)) is considered MEDICALLY NECESSARY in adult individuals with one of the following:
- Family history of first-degree relatives with premature atherosclerotic cardiovascular disease (ASCVD) (less than 55 years of age in men; less than 65 years of age in women) or elevated Lp(a)
- Individuals with premature ASCVD (less than 55 years of age in men; less than 65 years of age in women), particularly in the absence of traditional risk factors
- Individuals with primary severe hypercholesterolemia (LDL-C greater than or equal to 190 mg/dL) or suspected familial hypercholesterolemia (FH)
- Individuals at very high risk of ASCVD to better define those who are more likely to benefit from PCSK9 inhibitor therapy.
- High-sensitivity C-Reactive Protein (hs-CRP)
- Testing for high-sensitivity C-reactive protein (hs-CRP) is considered MEDICALLY NECESSARY if, after quantitative risk assessment using ACC/AHA Pooled Cohort Equations to calculate 10-year risk of CVD events (see Note 1), a risk-based treatment decision is uncertain.
The following does not meet coverage criteria due to a lack of available published scientific literature confirming that the test(s) is/are required and beneficial for the diagnosis and treatment of a patient’s illness.
-
- Testing for hs-CRP is investigational/unproven therefore considered NOT MEDICALLY NECESSARY for all other indications, including
- Use as a screening test for the general population
- For monitoring response to therapy
- Testing for hs-CRP is investigational/unproven therefore considered NOT MEDICALLY NECESSARY for all other indications, including
- High-sensitivity Cardiac Troponin
Measurement of High-sensitivity cardiac troponin T (hs-cTnT) is investigational/unproven therefore considered NOT MEDICALLY NECESSARY for cardiovascular risk assessment and stratification in the outpatient setting.
- Homocysteine
Homocysteine testing for cardiovascular disease risk assessment screening, evaluation, and management is investigational/unproven therefore considered NOT MEDICALLY NECESSARY. Homocysteine testing for indications other than CVD is addressed in Avalon Policy AHS-M2141-Testing of Homocysteine Metabolism-Related Conditions and AHS-G2014-Vitamin B12 and Methylmalonic Acid Testing.
(Rationale note - Avalon covers other alternative tests (e.g., lipid panel) which are widely used and equally valuable in CV risk assessment)
- Novel Cardiovascular Biomarkers
Measurement of novel lipid and non‒lipid biomarkers (e.g., apolipoprotein AI, apolipoprotein E, B-type natriuretic peptide, cystatin C, fibrinogen, leptin, LDL subclass, HDL subclass) is investigational/unproven therefore considered NOT MEDICALLY NECESSARY as an adjunct to LDL cholesterol in the risk assessment of cardiovascular disease.
- Cardiovascular Risk Panels
Cardiovascular risk panels, consisting of multiple individual biomarkers intended to assess cardiac risk (other than simple lipid panels, see Policy Guidelines below), is investigational/unproven therefore considered NOT MEDICALLY NECESSARY.
- Serum Intermediate Density Lipoprotein
Measurement of serum intermediate density lipoproteins is investigational/unproven therefore considered NOT MEDICALLY NECESSARY as an indicator of cardiovascular disease risk.
- Lipoprotein-associated Phospholipase A2
Measurement of lipoprotein-associated phospholipase A2 (Lp-PLA2) is investigational/unproven therefore considered NOT MEDICALLY NECESSARY as an indicator of risk of cardiovascular disease.
- Long-chain Omega-3 Fatty Acid
Measurement of long-chain omega-3 fatty acids in red blood cell membranes, including but not limited to its use as a cardiac risk factor is investigational/unproven therefore considered NOT MEDICALLY NECESSARY.
- All other tests for assessing CHD risk is investigational/unproven therefore considered NOT MEDICALLY NECESSARY.
Note 1:
2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk (Goff et al., 2014):
Risk factors include gender, age, race, smoking, hypertension, diabetes, total cholesterol, high- and low-density lipoprotein cholesterol. A race- and sex-specific PCE ASCVD Risk Estimator is available at: https://tools.acc.org/ldl/ascvd_risk_estimator/index.html#!/calulate/estimator/.
The 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol affirms that “the PCE is a powerful tool to predict population risk, but it has limitations when applied to individuals.” Hence a clinician-patient risk discussion can individualize risk status based on PCE, but with the inclusion of additional risk-enhancing factors. These additional factors may include:
- A family history of premature ASCVD (males, age < 55 y; females, age < 65 y)
- Primary hypercholesterolemia (LDL-C, 160 – 189 mg/dL [4.1 – 4.8 mmol/L); non-HDL-C 190 – 219 mg/dL [4.9 – 5.6 mmol/L])
- Metabolic syndrome (increased waist circumference, elevated triglycerides [>150 mg/dL], elevated blood pressure, elevated glucose, and low HDL-C [< 40 mg/dL in men; < 50 in women mg/dL] are factors; tally of 3 makes the diagnosis)
- Chronic kidney disease (eGFR 15 – 59 mL/min/1.73 m2 with or without albuminuria; not treated with dialysis or kidney transplantation)
- Chronic inflammatory conditions such as psoriasis, RA, or HIV/AIDS
- History of premature menopause (before age 40 y) and history of pregnancy-associated conditions that increase later ASCVD risk such as preeclampsia
- High-risk race/ethnicities (e.g., South Asian ancestry)
- Lipid/biomarkers: Associated with increased ASCVD risk
- Persistently elevated, primary hypertriglyceridemia (≥ 175 mg/dL)
- Elevated high-sensitivity C-reactive protein (≥ 2.0 mg/L)
- Elevated Lp(a): A relative indication for its measurement is family history of premature ASCVD. An Lp(a) ≥ 50 mg/dL or ≥ 125 nmol/L constitutes a risk-enhancing factor especially at higher levels of Lp(a)
- Elevated apoB ≥ 130 mg/dL: A relative indication for its measurement would be triglyceride ≥ 200 mg/dL. A level ≥ 130 mg/dL corresponds to an LDL-C ≥ 160 mg/dL and constitutes a risk-enhancing factor
- ABI < 0.9
Policy Guidelines:
A simple lipid panel is generally composed of the following lipid markers:
- Total cholesterol
- LDL cholesterol
- HDL cholesterol
- Triglycerides
Certain calculated ratios, such as the total/HDL cholesterol may also be reported as part of a simple lipid panel.
Other types of lipid testing, i.e., apolipoproteins, lipid particle number or particle size, lipoprotein (a), etc., are not considered to be components of a simple lipid profile.
Table of Terminology
|
Term |
Definition |
|
AA |
Arachidonic acid |
|
AACE |
American Association of Clinical Endocrinologists |
|
AACVPR |
American Association of Cardiovascular and Pulmonary Rehabilitation |
|
AAPA |
American Academy of Physician Assistants |
|
ABC |
Association of Black Cardiologists |
|
ABI |
Ankle-brachial index |
|
ACC |
American College of Cardiology |
|
ACCF |
American College of Cardiology Foundation |
|
ACE |
American College of Endocrinology |
|
ACPM |
American College of Preventive Medicine |
|
ADA |
American Diabetes Association |
|
ADMA |
Asymmetric dimethylarginine |
|
AGS |
American Geriatrics Society |
|
AHA |
American Heart Association |
|
Anti-CCP |
Anti–cyclic citrullinated peptide |
|
APhA |
American Pharmacists Association |
|
Apo A-I |
Apolipoprotein A-I |
|
Apo B |
Apolipoprotein B |
|
Apo C-III |
Apolipoprotein C-III |
|
Apo E |
Apolipoprotein E |
|
APs |
Antipsychotics |
|
ART |
Antiretroviral therapy |
|
ASCP |
American Society for Clinical Pathology |
|
ASCVD |
Atherosclerotic cardiovascular disease |
|
ASH |
American Society of Hypertension |
|
ASPC |
American Society for Preventive Cardiology |
|
BMI |
Body mass index |
|
BNP |
Brain natriuretic peptide |
|
CAC |
Coronary artery calcium |
|
CAC |
Coronary artery calcification |
|
CAD |
Coronary artery disease |
|
C-CHANGE |
Canadian Cardiovascular Harmonized National Guidelines Endeavour |
|
CCS |
Canadian Cardiovascular Society |
|
CDC |
Centers for Disease Control and Prevention |
|
CHARGE |
Cohorts for Heart and Aging Research in Genomic Epidemiology |
|
CHD |
Coronary heart disease |
|
CIMT |
Carotid intima media thickness |
|
CK |
Creatine kinase |
|
CKD |
Chronic kidney disease |
|
CRP |
C reactive protein |
|
cTnl |
Cardiac troponin l |
|
cTnT |
Cardiac troponin T |
|
CVD |
Cardiovascular disease |
|
DHA |
Docosahexaenoic acid |
|
DM |
Diabetes mellitus |
|
DoD |
Department of Defense |
|
DPA |
Docosapentaenoic acid |
|
EACPR |
European Association for Cardiovascular Prevention & Rehabilitation |
|
EAS |
European Atherosclerosis Society |
|
EASD |
European Association for the Study of Diabetes |
|
EEG |
Electroencephalography |
|
ELISA |
Enzyme-linked immunosorbent assay |
|
EPA |
Eicosapentaenoic acid |
|
ES |
Endocrine Society |
|
ESC |
European Society of Cardiology |
|
FDA |
Food and Drug Administration |
|
FH |
Familial hypercholesterolemia |
|
Fix |
Family history |
|
GDMT |
Guideline-directed medical therapy |
|
GFR |
Glomerular filtration rate |
|
GGT |
Gamma-glutamyltransferase |
|
GlycA |
Glycoprotein acetyls |
|
HDL |
High-density lipoprotein |
|
HDL-C |
High-density lipoprotein cholesterol |
|
HF |
Heart failure |
|
HIV |
Human immunodeficiency virus |
|
HRs |
Hazard ratios |
|
hsCRP |
High-sensitivity C reactive protein |
|
hs-cTnI |
High-sensitivity cardiac troponin i |
|
hs-cTnT |
High-sensitivity cardiac troponin t |
|
IDL |
Intermediate density lipoproteins |
|
LDL |
Low-density lipoprotein |
|
LDL cholesterol |
Low-density lipoprotein cholesterol |
|
LDL-C |
Low-density lipoprotein cholesterol |
|
LDTs |
Laboratory developed tests |
|
LMBP |
Laboratory Medicine Best Practices |
|
Lp(a) |
Lipoprotein A |
|
LPA |
Apolipoprotein(a) locus |
|
Lp-PLA2 |
Lipoprotein-associated Phospholipase A2 |
|
LSP |
Lipids Standardization Program |
|
MACE |
Major adverse cardiovascular event |
|
MESA |
Multi-Ethnic Study of Atherosclerosis |
|
NICE |
National Institute for Health and Care Excellence |
|
NLA |
National Lipid Association |
|
NMA |
National Medical Association |
|
Non-HDL-C |
Non-high-density lipoprotein cholesterol |
|
NT-proBNP |
Amino-terminal pro-B-type natriuretic peptide |
|
PAD |
Peripheral artery disease |
|
PCEs |
Pooled cohort equations |
|
PCNA |
Preventive Cardiovascular Nurses Association |
|
proBNP |
Pro-B-type natriuretic peptide |
|
RR |
Risk ratio |
|
sdLDL |
Small dense low-density lipoprotein |
|
SDMA |
Symmetric dimethylarginine |
|
SIGN |
Scottish Intercollegiate Guidelines Network |
|
SMC™ |
Single Molecule Counting |
|
T1DM |
Type 1 diabetes mellitus |
|
T2DM |
Type 2 diabetes mellitus |
|
TC |
Total cholesterol |
|
TG |
Triglyceride |
|
TG |
Total glycerides |
|
USPSTF |
U.S. Preventive Services Task Force |
|
VA |
Veterans Affairs |
|
VLDL |
Very low-density lipoprotein |
Rationale
Statistics show that cardiovascular disease (including coronary heart disease, stroke, and hypertension) is America's leading health problem, and the leading cause of death. According to the 2020 update of the heart disease and stroke statistics report released by the American Heart Association:
- Approximately 121 million people in this country suffer from some form of cardiovascular disease (encompassing coronary heart disease, heart failure, hypertension, and stroke).
- The direct and indirect costs of cardiovascular disease and stroke are about $351.3 billion and increasing every year.
- An estimated 18 million U.S. adults have coronary heart disease, 116 million U.S. adults have high blood pressure, and 26 million have diabetes.
- Heart failure affects over 6 million U.S. adults.
- On average, someone in the U.S. suffers a stroke every 40 seconds.
- Women have a higher lifetime risk of stroke than men.
- Approximately 15 percent of U.S. adults smoke cigarettes “some days” or “every day”.
- An estimated 68 percent of U.S. adults are overweight or obese (Virani Salim et al., 2020).
Cardiovascular Risk Assessment
Traditionally, the most important indicators for cardiac risk are those of a person's health history. These include factors such as family history, age, weight, exercise, and cigarette smoking status (Wilson, 2020b).
Tests typically used to assess cardiovascular risk include:
- Lipid profile or panel, which is the most important blood test for cardiac risk assessment
- Biomarkers
- Cardiovascular Risk Panels
Lipid Profile or Panel
A lipid profile or lipid panel is a panel of blood tests that serves as an initial broad medical screening tool for abnormalities in lipids, such as cholesterol and triglycerides. The results of this test can identify certain genetic diseases and can determine approximate risks for cardiovascular disease and other diseases. The lipid profile typically includes measurements of low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, and total cholesterol. Using these values, a laboratory may also calculate the very low-density lipoprotein (VLDL) and total cholesterol/HDL cholesterol ratio (Robert Rosenson, 2022).
Biomarkers
Traditional risk algorithms may miss up to 20% of cardiovascular disease (CVD) events (MacNamara et al., 2015). Numerous biomarkers have been proposed as potential risk markers for CVD. These biomarkers include but are not limited to several apolipoproteins (A, B, AI, E, LDL, HDL), B-type natriuretic peptide, and C-reactive protein. These biomarkers have been proposed as an alternative or addition for risk stratification in CVD or as treatment targets for lipid-lowering therapy (R Rosenson, 2022b, 2022d; Wilson, 2020a). However, even the most promising biomarkers have only demonstrated modest associations and predictive ability.
Antonopoulos et al. (2022) investigated the added prognostic value of biomarkers of vascular inflammation for stable patients without known coronary heart disease on top of clinical risk factors. The biomarkers — "C-reactive protein, interleukin-6 and tumor necrosis factor-a, arterial positron emission tomography/computed tomography and coronary computed tomography angiography-derived biomarkers of vascular inflammation, including anatomical high-risk plaque features and perivascular fat imaging” — were evaluated against the main endpoint of the difference in c-index (Δ[c-index]) with the use of inflammatory biomarkers for major adverse cardiovascular events (MACEs) and mortality, finding that the “Biomarkers of vascular inflammation provided added prognostic value for the composite endpoint and for MACEs only (pooled estimate for Δ[c-index]% 2.9, 95% CI: 1.7 – 4.1 and 3.1, 95% CI: 1.8 – 4.5, respectively)”. Coronary computed tomography angiography-related biomarkers are noteworthy as they produced the highest added prognostic benefit for MACEs, the biomarkers of which included “high-risk plaques 5.8%, 95% CI: 0.6 to 11.0, and perivascular adipose tissue (on top of coronary atherosclerosis extent and high-risk plaques): 8.2%, 95% CI: 4.0 to 12.5)”. Though this may appear to be the case, the study remarked that the net clinical benefit and cost-effectiveness of using these biomarkers are still underreported and understudied (Antonopoulos et al., 2022).
Since low-grade inflammation has been linked to early development of cardiovascular disease in the young, Chiesa et al. (2022) evaluated whether circulating levels of glycoprotein acetyls (GlycA) were better able to predict the development of adverse cardiovascular disease risk profiles when compared with the more commonly used biomarker high‐sensitivity CRP (C‐reactive protein). Using data from a total of 3306 adolescents and young adults from the Avon Longitudinal Study of Parents and Children (mean age, 15.4 ± 0.3; n = 1750) and Cardiovascular Risk in Young Finns Study (mean age, 32.1 ± 5.0; n = 1556), the authors found that not only did “GlycA showed greater within‐subject correlation over 9‐to‐10‐year follow‐up [for hypertension and metabolic syndrome] in both cohorts compared with CRP, particularly in the younger adolescent group (r = 0.36 versus 0.07)”, but GlycA was associated with multiple lifestyle‐related cardiovascular disease risk factors, cardiometabolic risk factor burden, and vascular dysfunction. Moreover, in both cohorts, “only GlycA predicted future risk of both hypertension (risk ratio [RR], ≈ 1.1 per z‐score increase for both cohorts) and metabolic syndrome (RR, ≈ 1.2 – 1.3 per z‐score increase for both cohorts) in 9‐to‐10‐year follow‐up”, suggesting that GlycA “may capture distinct sources of inflammation in the young and may provide a more sensitive measure than CRP for detecting early cardiovascular risk” (Chiesa et al., 2022).
Apolipoprotein B (Apo B)
Apo B is a major protein in the construction and regulation of lipids. There are two forms of apo B, apo B-48 and apo B-100. Apo B-100 is the major protein found in LDL and VLDL. Each LDL particle has one molecule of Apo B-100 per particle. Therefore, the apo B concentration may represent the amount of LDL well (R Rosenson, 2022a, 2022b; Robert Rosenson, 2022). Increased levels of apo B have been associated with atherosclerosis development in several large-scale studies; however, apo B levels have yet to become routinely measured in clinical practice (Morita, 2016; Trompet et al., 2018).
Researchers have hypothesized that lowering apo B levels in young or middle-aged individuals will reduce the number of atherosclerosis cases later in life (Robinson et al., 2018). Further, atherosclerotic changes in retinal arteries have been associated with coronary artery disease (CAD) as well as apo B, TG, TC, and LDL-C levels (Tedeschi-Reiner et al., 2005). Lamprea-Montealegre et al. (2020) have analyzed data from 9270 participants with chronic kidney disease to determine if triglyceride-rich lipoproteins contribute to a greater CVD risk in this population; it was determined that increased apo B along with other triglyceride and cholesterol-related concentrations were associated with an increased risk for atherosclerotic CVD risk in chronic kidney disease patients. A second study (n = 8570) has researched the relationship between apo B levels relative to LDL-C and non-LDL-C, as well as how these levels affect subclinical atherosclerotic cardiovascular disease (ASCVD) (Cao et al., 2019). Results showed that higher apo B levels were associated with an increase in coronary artery calcium (CAC) levels among adults older than 45 years who were not taking statins, “but provided only modest additional predictive value of apo B for CAC prevalence, incidence, or progression beyond LDL-C or non-HDL-C (Cao et al., 2019).” An equation to predict major cardiovascular events based on apo B levels has even been developed, and when studied, this equation showed major cardiovascular event “risk prediction comparable to directly-measured apo B in high risk patients with previous coronary heart disease (Hwang et al., 2017).”
The 2019 European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) published guidelines for the management of dyslipidaemias. These guidelines stated that “ApoB analysis is recommended for risk assessment, particularly in people with high TG [triglycerides], Diabetes mellitus (DM), obesity or metabolic syndrome, or very low LDL-C (Mach et al., 2019).” The ESC and EAS justify these recommendations by stating that the measurement of LDL-C levels in patients with dyslipidaemia may be inaccurate due to high DM or TG levels. “Because apo B provides an accurate estimate of the total concentration of atherogenic particles under all circumstances, it is the preferred measurement to further refine the estimate of ASCVD risk that is modifiable by lipid-lowering therapy (Mach et al., 2019).”
Apolipoprotein A-I (Apo-A-I)
Apo A-I is a lipid-binding protein which comprises HDL molecules. HDL contains two associated apolipoproteins, A-I and A-II, and together they are the primary components of the HDL molecules. Due to Apo A-I’s role as a primary structural protein for HDL, it significantly factors into the density ranges of HDL, which ultimately contribute to their overall measurement (R Rosenson, 2022b).
Direct measurement of apo A–I has been proposed as more accurate than the traditional use of HDL level. Low levels of apo A–I may be associated with an increased risk for CVD. Testing for apo A–I is often performed with apolipoprotein B and reported as a ratio (apo B: apo A-I), thus providing a measure of atherogenic to antiatherogenic lipoprotein particles (Sandhu et al., 2016).
Apolipoprotein E (Apo E)
Apo E is the primary apolipoprotein found in VLDLs and chylomicrons. Apo E is essential in the metabolism of cholesterol and triglycerides and helps to clear chylomicrons and VLDL. Apo E polymorphisms have functional effects on lipoprotein metabolism. Some Apo E genotypes are more atherogenic than others, and their measurement could provide additional information of risk of coronary artery disease (R Rosenson, 2022a, 2022b).
B-type or Brain Natriuretic Peptide (BNP)
BNP is a hormone released by the ventricles of the heart when pressure to the cardiac muscles increases or there is volume overload. BNP is now an established biochemical marker for heart failure, as the level of BNP in plasma increases proportionally based on disease severity (Kuwahara et al., 2018). Further, BNP has been accepted as an “independent surrogate marker of rehospitalization and death” for heart disease (Li & Wang, 2005), and exhibits both diagnostic and prognostic capabilities (Tomcsányi et al., 2018).
While BNP has shown great promise for diagnostic congestive heart failure purposes, a BNP guided heart failure treatment strategy seems to be controversial; some report that this type of treatment has led to greater health-related costs and does not increase patient outcomes (Mark et al., 2018). Still, many drugs, such as beta blockers, amiodarone, spironolactone, and angiotensin converting enzyme inhibitors, have been beneficial in reduction of circulating BNP during the management of chronic heart failure (Li & Wang, 2005). A major limitation of BNP is that a wide range of values are observed in patients with and without heart failure; for example, obese individuals tend to have lower levels of this hormone than healthy individuals (Colucci & Chen, 2022).
Januzzi et al. (2019) used data from the GUIDing Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT) trial to develop a greater understanding of the prognostic capabilities of amino-terminal pro-B-type natriuretic peptide (NT-proBNP) following heart failure. A total of 638 individuals participated in the study. The authors concluded that “Patients with heart failure with reduced ejection fraction whose NT-proBNP levels decreased to ≤ 1,000 pg/ml during GDMT [guideline-directed medical therapy] had better outcomes” (Januzzi et al., 2019). These results highlight the potential for NT-proBNP to be used as a prognostic tool following heart failure.
High Density Lipoprotein (HDL)
Apart from apolipoprotein content (AI and AII), HDL can be classified by size (small and large), by density (HDL2, HDL3), and by electrical charge (pre-beta, alpha and pre-alpha). There has been substantial interest in evaluating whether HDL subclass testing can be used to provide additional information on cardiovascular risk compared to HDL alone. HDL levels have been noted to be inversely related to CVD risk and possibly even protective against CVD. However, there are still many questions about the relationship between HDL and CVD risk, such as whether HDL levels are causative of lower CVD risk (R. Rosenson, 2021; R Rosenson, 2022b).
Low Density Lipoprotein (LDL)
LDL proteins are a significant risk factor in predicting atherosclerosis. The mechanism of how LDL subclass particles impact risk of CVD has not been determined although many mechanisms have been proposed. Even though LDL cholesterol levels may be normal, an elevation of small, dense LDL particles may be associated with CVD. One theory is that the small LDL particles can be more easily deposited into the intima, lead to atherosclerosis, and eventually CVD. Another is that LDL particles may upregulate the angiotensin II receptor, thereby promoting atherosclerosis (R Rosenson, 2022a, 2022b).
Lipoprotein(a)
Lipoprotein(a) (Lp[a]) is a low-density lipoprotein and has been determined to have atherogenic potential. Lp(a) has been proposed as an independent risk factor for coronary artery disease (CAD). Although research has shown it accumulates in atherosclerotic lesions, the actual process remains unclear. Serum levels of Lp(a) are highly determined by genetic polymorphisms, and the 90th percentile of Lp(a) levels was estimated at about 39 mg/dL. The overall degree of risk associated with Lp(a) levels appears to be modest, and the degree of risk may be mediated by other factors such as LDL levels and/or hormonal status. The standard method for measuring Lp(a) is density gradient ultracentrifugation. Although enzyme-linked immunosorbent assay (ELISA) techniques are available; they unable to distinguish between apo(a) isoforms, leading to inaccurate results (R Rosenson, 2022c). Lp(a) may have prognostic value in certain situations, such as in women with hypercholesterolemia (Grundy et al., 2019).
A study focusing on the possible role of Lp(a) in CVD was performed by Willeit et al. (2018); 26069 subjects were analyzed, and the authors found a linear relationship between elevated Lp(a) levels and CVD risk at a baseline of ≥30 mg/dL and an on-statin level of ≥ 50 mg/dL. The baseline hazard ratios were 1.13 and 1.36 for 30 – 50 mg/dL and > 50 mg/dL respectively, and the hazard ratios for patients on statins were 1.08 and 1.42 respectively (Willeit et al., 2018).
Mehta et al. (2020) investigated “independent and joint associations of Lp(a) and FHx [family history of coronary heart disease] with atherosclerotic cardiovascular disease (ASCVD) and CHD [coronary heart disease] among asymptomatic subjects.” A total of 12149 patients were included and observed over 21 years. The median age of this cohort was 54 years, and 44% of these patients had FHx. A total of 3114 ASCVD events were observed. Both FHx and elevated Lp(a) were independently associated with ASCVD, with a hazard ratio of 1.17 for FHx and a hazard ratio of 1.25 for elevated Lp(a). Patients with both FHx and elevated Lp(a) were found to have a hazard ratio of 1.43. Similar findings were found for CHD. The authors also noted that ASCVD and CHD risk reclassification and discrimination indices had improved accuracy with both FHx and Lp(a) included. The authors concluded that “elevated plasma Lp(a) and FHx have independent and additive joint associations with cardiovascular risk and may be useful concurrently for guiding primary prevention therapy decisions” (Mehta et al., 2020).
Cystatin C
Cystatin C is a protease inhibitor protein that plays a role in inflammation and obesity. Serum testing has been proposed to diagnose impaired kidney function, which in turn may be a risk factor for coronary heart disease (Rule, 2020). There is no published literature proving the effectiveness of Cystatin C as a biomarker for predicting cardiovascular risk and other confounding factors such as inflammation levels still need to be parsed out from Cystatin C. Overall, Cystatin C is not routinely used as a CVD biomarker (Sarnak, 2019).
Fibrinogen
Fibrinogen is a circulating glycoprotein that plays an important role in platelet aggregation and blood viscosity. Fibrinogen has been suggested as a possible indicator of inflammation that accompanies atherosclerosis. The independent predictive power, impact on management strategies, and clinical utility of fibrinogen measurement have shown conflicting results. One study of 150000 subjects demonstrated a log-linear relationship of fibrinogen and cardiovascular events, but another study of 90000 subjects did not find a relationship; therefore further research is required (Wilson, 2017, 2020b). A recent study has reported that higher fibrinogen levels increased the risk of a stroke in large arteries or small vessels but decreased the risk of cardioembolic stroke (Maners et al., 2019).
Pieters et al. investigated the contribution of fibrinogen, as well as other biomarkers, on cardiovascular disease (CVD) mortality. A total of 4487 patients were evaluated over a period of 14 years. The authors noted that 551 patients had CVD at baseline and over the time period investigated, 321 CVD deaths occurred. Fibrinogen was found to associate (“cluster”) with C-Reactive protein only and was associated with both baseline CVD and CVD mortality at follow-up. Both fibrinogen and gamma-glutamyl transferase were found to be mediators between CVD status and all-cause mortality, as well as between CVD status and CVD mortality (Pieters et al., 2020).
Leptin
Leptin is a protein secreted by fat cells and plays a role in fat metabolism. As leptin increases with obesity, it is thought to be associated with CVD. Leptin may play a role in regulating blood pressure, insulin sensitivity, inflammatory vascular responses, and more. However, a meta-analysis covering 13 studies, 4257 CVD patients, and 26710 controls indicated no significant relationship between leptin and CVD or stroke once other cardiovascular risk factors were controlled. The authors recommend further research to evaluate the effectiveness of leptin as a predictor of CVD (Wilson, 2017, 2020b; Yang et al., 2017). A recent study found that, in a Chinese cohort, serum leptin levels were identified as a marker for patients with first-ever acute ischemic stroke and were also associated with stroke size and severity (Liu et al., 2019).
Drug Therapies Requiring Lipid Monitoring
Lipid-lowering Therapy with Statins
Statins, such as ezetimibe, are a type of drug often prescribed to lower lipid levels or cholesterol. Pignone (2021) has reported that statins may reduce CVD risk by 20 to 30%, regardless of initial LDL-C levels. Statins are also beneficial for the treatment of arterial stiffness, independent of their hypolipidemic effect; treatment with a high dosage of statins will decrease LDL-C levels and improve arterial stiffness levels (Reklou et al., 2020). Kongpakwattana et al. (2019) report that the use of statin therapy in combination with non-stain lipid-modifying agents is more beneficial to reduce CVD risk than using only one treatment method.
A meta-analysis of statin trials completed by Boekholdt et al. (2014) analyzed data from 38,153 patients. During the follow-up of only 5,387 patients, it was found that 6,286 major cardiac events occurred. Great variability was recorded in LDL-C, apo B and non-HDL-C levels based on fixed statin levels over a one-year period. “Among trial participants treated with high-dose statin therapy, > 40% did not reach an LDL-C target < 70 mg/dl,” suggesting that high-dose statin therapy effectiveness may depend on the individual (Boekholdt et al., 2014).
Antipsychotics
Several atypical antipsychotic medications, such as risperidone, sertindole and olanzapine, have been FDA approved for the treatment of psychiatric disorders, including bipolar disorder, depression, and schizophrenia; unfortunately, these medications may lead to a plethora of side effects, including dyslipidemia, hypertension, increased CVD risk, obesity, sudden cardiac death, and insulin resistance (Beauchemin et al., 2019; Polcwiartek et al., 2016). Specifically, antipsychotic-induced corrected QT prolongation may increase the risk of Torsades de Pointes (a form of polymorphic ventricular tachycardia), leading to sudden cardiac death (Polcwiartek et al., 2016). While newer antipsychotics have been improved to lessen the pro-arrhythmic impact of their predecessors, they may contribute to cardiac death in a new way: by worsening the metabolic profile (Howell et al., 2019). It is recommended that any individuals in need of antipsychotics seriously consider the risks of these medications before accepting this type of treatment.
A 10-year study compared the CVD risk of patients with schizophrenia taking antipsychotics with healthy controls. The overall CVD risk was 5.16% in patients with schizophrenia, and 3.02% in the healthy control group; further, risk scores were significantly higher and HDL levels were significantly lower in patients taking multiple antipsychotics (Kilicaslan et al., 2019). A recent meta-analysis by Rotella et al. (2020) aimed to identify the long-term metabolic and cardiovascular effects of antipsychotic drugs. A total of 3013 studies were screened, and 92 were used for data analysis. The researchers have found a significantly higher risk of CVD death for sertindole users compared to risperidone users and state that “Long-term cardiovascular effects of APs [antipsychotics] deserve to be studied more extensively (Rotella et al., 2020).”
Accutane
Accutane, also known as isotretinoin, is a synthetic vitamin A derivative and oral medication often prescribed for the treatment of severe acne; it was approved by the FDA in 1982 to treat resistant, nodular acne that has not responded to conventional therapeutic measures such as systemic antibiotics (Pile & Sadiq, 2019). Unfortunately, isotretinoin therapy may cause various cardiac events, including congenital heart disease, atrial tachycardia, and cardiac remodeling (Guler et al., 2015). Akcay and Yuksel (2019) have reported that isotretinoin use may have been related to the development of Kounis syndrome (acute coronary syndrome due to a reduction of blood flow to the heart) in one patient. Alan et al. (2016) reported that isotretinoin use may have triggered premature ventricular contractions in a 33-year-old woman. Karadag et al. (2012) completed a study comprised of 70 patients who were being treated with 0.5 – 1.0 mg/kg per day of isotretinoin; in each patient, heart rate, blood pressure, EEG, biochemical and hematologic parameters were all measured. “We found that isotretinoin did not affect P- and QT-wave measurement (Karadag et al., 2012).”
Isotretinoin may also affect serum lipid levels. Zane et al. (2006) studied 13772 patients with acne currently receiving oral isotretinoin therapy. Results showed that 31% of isotretinoin users had high cholesterol levels, 11% had high liver transaminase levels, and 44% had high triglyceride levels (Zane et al., 2006). In a more recent study, Lee et al. (2016) completed a systematic review and meta-analysis from 1960 – 2013 which studied the effects of oral isotretinoin use. Data was only admitted if 40 mg/day of isotretinoin was used for at least four weeks. The authors stated that “This meta-analysis showed that (1) isotretinoin is associated with a statistically significant change in the mean value of several laboratory tests (white blood cell count and hepatic and lipid panels), yet (2) the mean changes across a patient group did not meet a priori criteria for high-risk and (3) the proportion of patients with laboratory abnormalities was low (Lee et al., 2016; Zane et al., 2006).” The authors concluded by stating that these results do not support monthly laboratory testing for patients taking standard isotretinoin doses for acne purposes.
Other Cardiovascular Markers
C Reactive Protein (CRP)
Data from numerous studies have shown an association between elevated serum or plasma concentrations of CRP and atherosclerotic vascular disease, risk of recurrent cardiovascular events, and the incidence of initial cardiovascular events among individuals not known to have atherosclerosis (Crea, 2020).
CRP can be measured using either traditional assays or high sensitivity CRP (hs-CRP) assays. Traditional assays have limited use when screening for cardiovascular disease due to their limit of detection (3 – 5 mg/L). On the other hand, hs-CRP assays can detect concentrations of CRP down to 0.3 mg/L and below. These hs-CRP assays are used to assess cardiovascular risk because they can detect and quantify CRP within the range normally seen in asymptomatic patients (< 3 mg/L). Elevated CRP levels, either alone or in combination with other cardiovascular risk factors, have been associated with a higher risk of future cardiovascular events. Studies evaluating CRP in asymptomatic populations have shown that the baseline level of CRP predicts the long-term risk of a first myocardial infarction (MI), ischemic stroke, hypertension, peripheral vascular disease, sudden cardiac death, and all-cause mortality (Crea, 2020).
Homocysteine
Homocysteine is an amino acid that is produced by the body. Elevated levels of homocysteine may result in damage to the walls of the artery, increase the potential for thrombosis and lead to advanced atherosclerosis. Hence, elevated homocysteine levels have been demonstrated to increase the risk of CVD. However, the testing of homocysteine levels is not consistently recommended because, based on current research, the lowering of plasma homocysteine levels does not necessarily lower the risk of CVD. Further research is required to support the clinical utility of lowering homocysteine levels (R. S. Rosenson, Smith, C. Christopher, Bauer, Kenneth A., 2021).
Intermediate Density Lipoproteins (IDL)
Intermediate Density Lipoproteins (remnant cholesterol or lipoproteins) are the cholesterol content of triglyceride-rich lipoproteins, which is composed of VLDL and IDL in the fasting state, and is a combination of VLDL, IDL, and chylomicron remnants in the non-fasting state. It can be estimated by triglyceride (TG) levels in the absence of advanced lipoprotein testing. Elevated non-fasting plasma triglyceride is associated with increased risk for CVD (Varbo et al., 2013). Triglycerides are unlikely to directly cause CVD, thus VLDL and IDL are more commonly identified as the source of this increased risk for CVD (Jepsen et al., 2016). VLDL and IDL have been shown to be proatherogenic with both proinflammatory and prothrombotic effects (Joshi et al., 2016).
Genetic case studies have shown that elevated levels of remnant cholesterol are causally associated with both low-grade inflammation and CVD. Elevated levels of LDL cholesterol are associated with CVD, but not with low-grade inflammation. This indicates that elevated LDL cholesterol levels cause atherosclerosis without inflammation, whereas elevated remnant cholesterol levels lead to both atherosclerosis and inflammation (Varbo et al., 2014; Varbo et al., 2013).
Another measure which includes IDL is Non-HDL-C, which is derived from the simple calculation of total cholesterol minus HDL-C. The Emerging Risk Factors Collaboration concluded that apoB and non-HDL-C predicted risk similar to directly measured LDL-C and that fasting did not affect the hazard ratios (HRs) (Di Angelantonio et al., 2009).
Lipoprotein-associated Phospholipase A2 (Lp-PLA2)
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an inflammatory enzyme expressed in atherosclerotic plaques. It has been proposed that Lp– PLA2 testing may aid in detecting CVD risk due to its association with other biomarkers, such as LDL. The rationale for Lp-PLA2 as a key inflammatory biomarker is attractive because this enzyme is produced in atherosclerotic plaques with elevated expression found in CVD patients (Rosenson & Stafforini, 2012).
Numerous studies evaluate Lp-PLA2 as a predictor of cardiovascular risk (Garg et al., 2015; LPSC, 2010; Sudhir, 2006). These studies demonstrate that Lp-PLA2 is an independent predictor of CVD. Preliminary clinical trials of Lp-PLA2 inhibitors showed some improvements in physiologic measures, such as reduction in hs-CRP (Sudhir, 2006). However, further clinical trials of Lp-PLA2 inhibitors failed to demonstrate significant improvements in patient outcomes (Mohler et al., 2008). Although Lp-PLA2 does not appear to have any predictive power with apparently healthy individuals, it may have utility for symptomatic patients. The link between the enzyme and LDL is found in the enzyme’s plasma activity, which tends to vanish with treatment (Rosenson & Stafforini, 2012). De Stefano et al. (2019) stated that Lp-PLA2 may be considered as a new vascular specific biomarker to predict CVD in a population of patients with metabolic diseases.
Long-Chain Omega-3 Fatty Acids
Omega-3 fatty acids, a specific group of polyunsaturated fatty acids containing a double bond three carbons from the methyl terminus, are main building blocks of many fats and oils. Long-chain omega 3 fatty acids (≥C20, LC) include eicosapentaenoic acid (EPA, 20:5ω3), docosapentaenoic acid (DPA, 22:5ω3) and docosahexaenoic acid (DHA, 22:6ω3) and are thought to be beneficial in the prevention of coronary heart disease (CHD) (Mozaffarian, 2021). Circulating blood levels of EPA and DHA are inversely and significantly associated with reduced CHD event risk (de Oliveira Otto et al., 2013). Blood levels of omega-3 fatty acids may be more related to CVD benefit than the daily dose of fish oil supplements (Superko et al., 2014). The blood EPA/arachidonic acid (AA) ratio may be a clinically relevant measurement as AA has atherogenic and thrombogenic metabolites. Although this ratio has substantial individual variability, an EPA/AA ratio >0.75 has been associated with a significantly lower number of major coronary events in a Japanese population (Itakura et al., 2011). Determination of blood omega-3 levels may help guide the appropriate use of dietary fish or omega-3 supplements in a personalized heart disease prevention strategy.
The relationship of fish and dietary omega-3 fatty acids and cardiovascular disease (CVD) has been investigated in numerous studies and comprehensive reviews and recommendations exist, but guidance on blood concentrations is missing. Some prospective fish oil treatment investigations report a significant reduction in CVD events, but others do not (Bosch et al., 2012; Itakura et al., 2011). A meta-analysis did not find a statistically significant relationship between omega-3 consumption and CVD mortality (Rizos et al., 2012). A science advisory from the AHA stated that for individuals with prevalent CHD such as a recent MI event, treatment with omega-3 PUFA supplements is reasonable; further, for patients with prevalent heart failure without preserved left ventricular function, fish oil treatment is recommended, while treatment is not recommended for patients with diabetes mellitus, prediabetes or as a method for stroke prevention (Siscovick et al., 2017).
Troponins (I, T)
Troponins are specific biomarkers for cardiac injury and are often used to diagnose myocardial infarctions. These proteins control the calcium-mediated interaction of actin and myosin in the muscle, and the cardiac versions of these proteins are unique to the heart. There are two primary categories of tests for troponins; “sensitive or contemporary” and “high-sensitive.” The high-sensitive version is preferred due to its superior accuracy (Gibson, 2022; Jaffe, 2022, 2021).
Elevated levels of troponins are proposed to predict CVD risk. Ford et al. (2016) performed a study evaluating troponin levels in 3318 men in relation to CVD risk. A hazard ratio of 2.3 for the highest quartile of troponin (≥5.2 ng/L) compared to the lowest quartile (≤ 3.1 ng/L) was found. The authors also found a 5-fold reduction in coronary events when troponin levels decreased by a quarter (Ford et al., 2016).
Tang et al. evaluated the ability of high-sensitivity cardiac troponin I (hs-cTnI) to assess cardiovascular risk and mortality. A total of 5876 patients ages 66-90 years were included. A total of 1053 deaths (321 CVD-related) occurred, within a median follow-up of 6.3 years. Patients with an elevated hs-cTnI and without history of CVD had a similar mortality risk to patients with a CVD history but without an elevated hs-cTnI. However, after adjustment, elevated hs-cTnI was found to be associated with mortality risk, by a hazard ratio of 2.38 over low hs-cTnI and no CVD. Elevated hs-cTnI was found to be independently associated with incident CVD by a hazard ratio of 3.41, ASCVD (HR = 2.02) and heart failure (HR = 6.16). The authors concluded that “Hs-cTnI improves mortality and CVD risk stratification in older adults beyond traditional risk factors and improved model discrimination more than hs-cTnT for certain outcomes” (Tang et al., 2020)
Suthahar et al. “evaluate[d] associations of high-sensitivity cardiac troponin-T (cTnT) with cardiovascular disease (CVD), heart failure (HF), and mortality in community-dwelling women and men”. A total of 8226 adults were included in the study. The authors detected cTnT levels in 1102 women and 2396 men. The authors found these baseline levels to be associated with a greater risk of developing CVD in women compared to men (women hazard ratio = 1.48, men hazard ratio = 1.20). Similar sex-related differences were found for heart failure and mortality. Women at 6 ng/L were also found to have significantly increased risk for CVD, HF, and mortality whereas men were only found to have significantly increased risk for CVD at the same level of cTnT (Suthahar et al., 2020).
Proprietary Testing
Cardiovascular Risk Panels/Profiles
Cardiovascular risk panels refer to combinations of cardiac markers that are used for the risk assessment of developing cardiovascular disease, major adverse cardiovascular events, or ischemic cerebrovascular events. Commercially available risk panels use different combinations of lipids, inflammatory, genetic, and metabolic markers. Risk panels report the results of multiple individual tests, whereas quantitative risk scores generally use proprietary algorithms to combine the results of multiple markers into one score. The clinical utility of risk panels is lacking as the impact of results on patient management is unknown.
Examples of commercially available cardiovascular risk panels include, but are not limited to:
- Genova Diagnostics CV Health Plus™ Panel:
- Lipid markers (LDL; total cholesterol; HDL; triglycerides; LDL size; LDL particle number; HDL size; HDL particle number; lipoprotein (a))
- Independent risk factors (hs-CRP; fibrinogen; homocysteine; LP-PLA2)
- Insulin Resistance Score by Lipid Fractionation (LDL size; Small LDL particle number; HDL size; Large HDL particle number; Large very low-density lipoprotein (VLDL) particle number; VLDL size)
- Cleveland HeartLab CVD Inflammation Testing Profile
- F2-isoprostanes; oxidized LDL; hs-CRP; ADMA/SDMA; microalbumin; myeloperoxidase; Lp-PLA2 activity (HeartLab, 2020).
- Singulex® clinical lab test panels:
- Cardiac Function panel: SMC™ cTnl; NT-proBNP
- Vascular Inflammation panel: SMC™ Endothelin; SMC™IL-6; SMC™ IL-17A; SMC™ TNFα; Ferritin; homocysteine; Lp-PLA2; hs-CRP; uric acid; vitamin D; anti-CCP; rheumatoid factor; folate; vitamin B12.
- Lipid Management panel: Total Cholesterol, LDL-C (direct), apo A-1, apo B, sdLDL, HDL-C, HDL with fractionation (HDL + HDL2 + HDL3), triglycerides, Lp(a), total CK.
- Diabetes and Weight Management: Adiponectin, Cortisol, Cystatin C, Ferritin, GGT, Glucose, HbA1c, Insulin, Leptin, Vitamin D, Calcium, Magnesium, Phosphorus (Singulex, 2019). Singulex ceased operations as of June 2019 (360dx, 2019).
- WellnessFX various packages:
- Basic offerings include panels with markers such as Apo A-1 and Apo-B; the basic panel comes with over 25 markers, with the “premium” panel assessing 92 markers (WellnessFX, 2021).
American College of Cardiology (ACC) and the American Heart Association (AHA)
2019 ACC and AHA guidelines state that “Adults who are 40 to 75 years of age and are being evaluated for cardiovascular disease prevention should undergo 10-year atherosclerotic cardiovascular disease (ASCVD) risk estimation and have a clinician-patient risk discussion before starting on pharmacological therapy, such as antihypertensive therapy, a statin, or aspirin. In addition, assessing for other risk-enhancing factors can help guide decisions about preventive interventions in select individuals, as can coronary artery calcium scanning (Arnett et al., 2019).”
Laboratory testing was not addressed in this update.
The ACC and AHA published joint guidelines on the assessment of cardiovascular risk in asymptomatic patients in 2010 (Greenland et al., 2010), and updated in 2013 (Goff et al., 2014).
In adults between the ages of 20 and 79 who are free from CVD, the ACC/AHA state that it is reasonable to assess risk factors (smoking, hypertension, diabetes, total cholesterol, high density lipoprotein cholesterol) every four to six years so as to calculate 10-year CVD risk (Goff et al., 2014).
ACC/AHA also made the following recommendations on reclassification or contribution to risk assessment when high-sensitivity C-reactive protein (hs-CRP), apolipoprotein B (ApoB), glomerular filtration rate (GFR), microalbuminuria, family history, cardiorespiratory fitness, ankle-brachial index (ABI), carotid intima-media thickness (CIMT), or coronary artery calcium (CAC) score are considered in addition to the variables that are in the traditional risk scores:
- If, after quantitative risk assessment, a risk-based treatment decision is uncertain, assessment of 1 or more of the following—family history, hs-CRP, ABI or CAC may be considered to inform treatment decision making.
- CIMT is not recommended for routine measurement in clinical practice for risk assessment for a first ASCVD event.
- The contribution to risk assessment for a first ASCVD event using ApoB, chronic kidney disease, albuminuria, or cardiorespiratory fitness is uncertain at present (Goff et al., 2014).
The 2010 guidelines contained the following statement concerning testing for Lp-PLA2: Lipoprotein-associated phospholipase A2 might be reasonable for cardiovascular risk assessment in intermediate-risk asymptomatic adults. However, the 2013 guidelines on the assessment of cardiovascular risk do not mention Lp-PLA2 testing (Goff et al., 2014; Greenland et al., 2010).
The updated guidelines do not address arterial compliance, lipoprotein-associated phospholipase, long-chain omega-3 fatty acids, or endothelial function assessment as methods to assess initial CVD risk (Goff et al., 2014; Greenland et al., 2010).
The ACC notes cutoffs of certain biomarkers for increased ASCVD risk, which are as follows: persistently elevated, primary hypertriglyceridemia ≥ 175 mg/dL, ≥ 2 mg/L hs-CRP, ≥ 50 mg/dL or ≥ 125 nmol Lp(a), ≥ 130 mg/dL Apo B (corresponding to > 160 mg/dL LDL-C), and < 0.9 ankle-brachial index (ABI) (ACC, 2018; Grundy et al., 2019).
The ACC and AHA also released joint guidelines with the AAPA, ABC, ACPM, AGS, APhA, ASH, ASPC, NMA, and PCNA, stating that screening and management of dyslipidemia/hypercholesterolemia is recommended in adults with hypertension (defined as > 130/80 mmHg) (Whelton et al., 2018).
2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines
This joint report discusses management of blood cholesterol. The report addresses treatments, populations of interest, and serum assessments of relevant cardiovascular biomarkers such as Apo B and lipoprotein A. The relevant recommendations are listed below:
The report notes that although measurement of Apo B may be “unreliable”, persistent elevation of Apo B may be considered a risk factor. The report remarks that a level of > 130 mg/dL Apo B should be considered a risk-enhancing factor [of ASCVD], as it corresponds to an LDL-C level of ≥ 160 mg/dL.
The report also remarks that Lp(a) is considered a risk factor for ASCVD at levels of “≥ 50 mg/dL or ≥ 125 nmol/L”. However, the authors write that it should be “considered in women only in the presence of hypercholesterolemia and with the understanding that the improvement in risk prediction in adult women in a large clinical trial was minimal”.
The power of these risk factors can be seen in the “pooled cohort equation”, “the single most robust tool for estimating 10-year risk in US adults 40 to 75 years of age.” These algorithms have strong representative power for larger populations. However, a notable limitation of these algorithms is that they are not as accurate for individuals. Hence a clinician-patient risk discussion can individualize risk status based on PCE, but with the inclusion of additional risk-enhancing factors. These additional factors may include:
- “A family history of premature ASCVD (males, age < 55 y; females, age < 65 y)
- Primary hypercholesterolemia (LDL-C, 160 – 189 mg/dL [4.1 – 4.8 mmol/L); non-HDL-C 190 – 219 mg/dL [4.9 – 5.6 mmol/L])
- Metabolic syndrome (increased waist circumference, elevated triglycerides [> 150 mg/dL], elevated blood pressure, elevated glucose, and low HDL-C [< 40 mg/dL in men; < 50 in women mg/dL] are factors; tally of 3 makes the diagnosis)
- Chronic kidney disease (eGFR 15 – 59 mL/min/1.73 m2 with or without albuminuria; not treated with dialysis or kidney transplantation)
- Chronic inflammatory conditions such as psoriasis, RA, or HIV/AIDS
- History of premature menopause (before age 40 y) and history of pregnancy-associated conditions that increase later ASCVD risk such as preeclampsia
- High-risk race/ethnicities (e.g., South Asian ancestry)
- Lipid/biomarkers: Associated with increased ASCVD risk
- Persistently elevated, primary hypertriglyceridemia (≥ 175 mg/dL)
- Elevated high-sensitivity C-reactive protein (≥ 2.0 mg/L)
- Elevated Lp(a): A relative indication for its measurement is family history of premature ASCVD. An Lp(a) ≥ 50 mg/dL or ≥ 125 nmol/L constitutes a risk-enhancing factor especially at higher levels of Lp(a)
- Elevated apoB ≥ 130 mg/dL: A relative indication for its measurement would be triglyceride ≥ 200 mg/dL. A level ≥ 130 mg/dL corresponds to an LDL-C ≥ 160 mg/dL and constitutes a risk-enhancing factor
- ABI < 0.9” (Grundy et al., 2019)
American Diabetes Association (ADA)
The updated ADA Standards of Medical Care in Diabetes document also includes a section focused on cardiovascular disease and risk management. Relevant guidelines and notes are captured below.
- “Blood pressure should be measured at every routine clinical visit. When possible, patients found to have elevated blood pressure (≥ 140/90 mmHg) should have blood pressure confirmed using multiple readings, including measurements on a separate day, to diagnose hypertension. Patients with blood pressure ≥ 180/110 mmHg and cardiovascular disease could be diagnosed with hypertension at a single visit.”
- “All hypertensive patients with diabetes should monitor their blood pressure at home.”
- “In asymptomatic patients, routine screening for coronary artery disease is not recommended as it does not improve outcomes as long as atherosclerotic cardiovascular disease risk factors are treated.”
- “Consider investigations for coronary artery disease in the presence of any of the following: atypical cardiac symptoms (e.g., unexplained dyspnea, chest discomfort); signs or symptoms of associated vascular disease including carotid bruits, transient ischemic attack, stroke, claudication, or peripheral arterial disease; or electrocardiogram abnormalities.”
- “Candidates for advanced or invasive cardiac testing include those with 1) typical or atypical cardiac symptoms and 2) an abnormal resting electrocardiogram (ECG). Exercise ECG testing without or with echocardiography may be used as the initial test. In adults with diabetes ≥ 40 years of age, measurement of coronary artery calcium is also reasonable for cardiovascular risk assessment. Pharmacologic stress echocardiography or nuclear imaging should be considered in individuals with diabetes in whom resting ECG abnormalities preclude exercise stress testing (e.g., left bundle branch block or ST-T abnormalities). In addition, individuals who require stress testing and are unable to exercise should undergo pharmacologic stress echocardiography or nuclear imaging.”
- “The screening of asymptomatic patients with high ASCVD risk is not recommended, in part because these high-risk patients should already be receiving intensive medical therapy, an approach that provides benefit similar to invasive revascularization.”
- “In adults not taking statins or other lipid-lowering therapy, it is reasonable to obtain a lipid profile at the time of diabetes diagnosis, at an initial medical evaluation, and every 5 years thereafter if under the age of 40 years, or more frequently if indicated.”
- “… risk scores and other cardiovascular biomarkers have been developed for risk stratification of secondary prevention patients (i.e., those who are already high risk because they have ASCVD) but are not yet in widespread use.”
- “The American College of Cardiology/American Heart Association ASCVD risk calculator (Risk Estimator Plus) is generally a useful tool to estimate 10-year risk of a first ASCVD event…The 10-year risk of a first ASCVD event should be assessed to better stratify ASCVD risk and help guide therapy, as described below.”
- “Obtain a lipid profile at initiation of statins or other lipid-lowering therapy, 4 – 12 weeks after initiation or a change in dose, and annually thereafter as it may help to monitor the response to therapy and inform medication adherence (ADA, 2020, 2021a).”
Also, for children and adolescents, the following recommendations were given for dyslipidemia testing:
- “Initial lipid testing should be performed when initial glycemic control has been achieved and age is ≥ 2 years. If initial LDL cholesterol is ≤ 100 mg/dL (2.6 mmol/L), subsequent testing should be performed at 9 – 11 years of age. Initial testing may be done with a nonfasting non-HDL cholesterol level with confirmatory testing with a fasting lipid panel.
- If LDL cholesterol values are within the accepted risk level (< 100 mg/dL [2.6 mmol/L]), a lipid profile repeated every 3 years is reasonable (ADA, 2020, 2021b).”
National Lipid Association (NLA)
The NLA published a scientific statement for lipid measurements in the management of cardiovascular disease, and their recommendations (with evidence rating of “B” or higher) are included below:
- “It is recommended to follow up abnormal screening lipid measurements with fasting lipid measurement (Strength: IIa. Evidence: B-NR)
- LDL-C in adults ≥ 190 mg/dL (≥160 mg/dl in children) is recommended to be reported as possible Familial Hypercholesterolemia (Strength: I. Evidence: B-NR)
- Non-HDL-C in adults ≥ 220 mg/dL is recommended to be reported as possible inherited hyperlipidemia (Strength: I. Evidence: B-NR)
- Triglyceride concentration ≥ 500 mg/dL is recommended to be reported as severe hypertriglyceridemia (Strength: I. Evidence: B-NR)
- Lipid measurements are recommended at 3 – 12 months for those on a stable medication regimen (Strength: I. Evidence: B-NR)
- Lipid measurements are recommended 4 – 12 weeks after a change in lipid treatment (Strength: I. Evidence: B-NR)
- LDL-C measurement is recommended for screening (Strength: I. Evidence: B-NR)
- LDL-C measurement is recommended on lipid therapy (Strength: I. Evidence: B-NR)
- Non-HDL-C measurement is recommended for screening (Strength: I. Evidence: B-NR)
- Non-HDL-C measurement is recommended on lipid therapy (Strength: I. Evidence: B-NR)
- Apolipoprotein B measurement may be reasonable for initial evaluation (Strength: IIb. Evidence: B-NR)
- Apolipoprotein B measurement is reasonable on lipid therapy (Strength: IIa. Evidence: B-NR)
- Apolipoprotein B measurement is recommended to facilitate diagnosis of Familial Dysbetalipoproteinemia and Familial Combined Hyperlipidemia (Strength: IIb. Evidence: B-NR)
- Lipoprotein (a) measurement is reasonable for initial evaluation in those with premature ASCVD, family history of premature ASCVD or of elevated Lp(a), history of LDL-C > 190 mg/dL or suspected FH, or those with very high ASCVD risk (Strength: IIa. Evidence: B-NR)
- Lipoprotein (a) measurement may be reasonable on lipid therapy to determine those who may benefit from PCSK9 therapy who are already on maximal dose statin therapy ± ezetimibe, whose LDL-C remains above 70 mg/dl (Strength: IIb. Evidence: B-NR)”
Wilson et al. (2019) published a scientific statement to provide an update on the use of lipoprotein A [Lp(a)] in the clinical setting, particularly for atherosclerotic cardiovascular disease (ASCVD).
The Association lists the following recommendations for Lp(a) testing in clinical practice:
For adults over 20 years old, “Measurement of Lp(a) is reasonable to refine risk assessment for ASCVD events in:
-
- Individuals with a family history of first-degree relatives with premature ASCVD (< 55 y[ears] of age in men, 65 y of age in women)
- Individuals with premature ASCVD (males aged < 55 y and females aged <65 y), particularly in the absence of traditional risk factors
- Individuals with primary severe hypercholesterolemia (LDL ≥ 190 mg/dL) or suspected FH [familial hypercholesterolemia]
- Individuals at very high** risk of ASCVD to better define those who are more likely to benefit from PCSK9 inhibitor therapy.”
**Very high risk is defined as “Individuals with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.”
The guidelines further remark that “Measurement of Lp(a) may be reasonable with:
- Intermediate (7.5% – 19.9%) 10-y ASCVD risk when the decision to use a statin is uncertain, to improve risk stratification in primary prevention.
- Borderline (5% – 7.4%) 10-y ASCVD risk when the decision to use a statin is uncertain, to improve risk stratification in primary prevention.
- Less-than-anticipated LDL-C lowering, despite good adherence to therapy.
- A family history of elevated Lp(a).
- Calcific valvular aortic stenosis.
- Recurrent or progressive ASCVD, despite optimal lipid-lowering therapy.”
Finally, the guidelines list recommendations for “youth” (< 20 years old), stating that “Measurement of Lp(a) may be reasonable with:
- Clinically suspected or genetically confirmed FH.
- Individuals with a family history of first-degree relatives with premature ASCVD (<55 y of age in men, 65 y of age in women)
- An unknown cause of ischemic stroke
- A parent or sibling found to have an elevated Lp(a) (Wilson et al., 2019).”
Centers for Disease Control and Prevention (CDC)
The CDC highlights the importance of cardiovascular disease biomarkers and has developed a reference laboratory and clinical standardization program to provide reference measurements for HDL-C, LDL-C, TG and total cholesterol (TC). The accuracy of the labs that analyze these biomarkers is also monitored by the CDC (CDC, 2017a).
The CDC notes that several health conditions increase the risk of heart disease including smoking, diabetes mellitus, obesity, high blood pressure, excessive alcohol use, physical inactivity, and unhealthy blood cholesterol levels. It is stated that “High blood cholesterol usually has no signs or symptoms. The only way to know whether you have high cholesterol is to get your cholesterol checked. Your health care team can do a simple blood test, called a “lipid profile,” to measure your cholesterol levels (CDC, 2019).”
The CDC has also developed the Lipids Standardization Program (LSP). This program ensures that the measurements reported in research studies and clinical laboratories are accurate. Blinded samples traceable to the CDC Reference Laboratory are provided to participants. The samples will be measured for total cholesterol (TC), glycerides (TG), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A-I (apo A-I), and apolipoprotein B (apo B). LSP participants report their results from the provided samples back to the CDC where these results are then analyzed; if results are accurate, those laboratories receive a certificate and are considered CDC-certified (CDC, 2017b).
American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE)
The 2017 AACE and ACE Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease recommend:
- Screening guidelines for dyslipidemia vary by age group;
- Although ASCVD risk in young adults is low, adults older than 20 years should be evaluated for dyslipidemia every 5 years as part of a global risk assessment
- Middle-aged individuals (Men 45 – 65 years, Women 55 – 65 years) should be screened for dyslipidemia at least every 1 to 2 years.
- All individuals with diabetes should be screened with a lipid profile at the time of diagnosis and annually thereafter. Some individuals with diabetes can be screened less frequently based on clinical considerations
- Annual screen for dyslipidemia for adults over 65 is recommended
- In children at risk for FH (e.g., family history of premature cardiovascular disease or elevated cholesterol), screening should be at 3 years of age, between 9 and 11, and at age 18
- Screen adolescents older than 16 years every 5 years or more frequently if they have ASCVD risk factors, have overweight or obesity, have other elements of the insulin resistance syndrome, or have a family history of premature ASCVD
- Direct measurement of LDL-C should be used to assess LDL-C in certain high-risk individuals, such as those with fasting TG concentrations greater than 250 mg/dL or those with diabetes or known vascular disease
- Apolipoproteins, Apo B and/or an apo B/apo A1 ratio calculation and evaluation may be useful in at-risk individuals.
- hsCRP is recommended to stratify ASCVD risk in individuals with a standard risk assessment that is borderline, or in those with an intermediate or higher risk with an LDL-C concentration < 130 mg/dL.
- Lp-PLA2 measurement, is recommended when it is necessary to further stratify an individual’s ASCVD risk, especially in the presence of hsCRP elevations.
- The routine measurement of homocysteine, uric acid, plasminogen activator inhibitor-1, or other inflammatory markers is not recommended because the benefit of doing so is not sufficiently proven.
- Coronary artery calcification (CAC) measurement has been shown to be of high predictive value and is useful in refining risk stratification
- Carotid intima media thickness (CIMT) may be considered to refine risk stratification (Jellinger et al., 2017).
The AACE/ACE published an updated algorithm in 2020. This algorithm focuses on “management of dyslipidemia and prevention of cardiovascular disease” and “complements” the above guidelines but includes information not available in 2017. Their relevant recommendations are listed below:
The guideline lists Apo B, LDL, Lp(a), and hs-CRP as biomarkers that may be “considered” in assessment of ASCVD risk for patients. The guideline also remarks that “measurement of apo B is useful in assessing the success of lipid-lowering therapy, since apo B may remain above goal after achieving the LDL-C goal.” Apo B is listed as a component of treatment goals, alongside LDL-C, Non-HDL-C, and TG [triglycerides].
The guideline recommends “considering” measurement of Lp(a) (lipoprotein A) in the following settings:
-
- “All patients with clinical ASCVD, especially premature or recurrent ASCVD despite LDL-C lowering;
- Individuals with a family history of premature ASCVD and/or increased Lp(a);
- Individuals with South Asian or African ancestry, especially with a family history of ASCVD or increased Lp(a);
- Individuals with a 10-year ASCVD risk ≥10% (primary prevention setting), in order to stratify risk;
- Patients with a personal or family history of aortic valve stenosis;
- Patients with refractory elevations of LDL-C despite aggressive LDL-C-lowering therapy (i.e., statin resistance)” (AACE, 2021).
The AACE also published a “consensus statement” on the “comprehensive type 2 diabetes management algorithm.” The guideline includes a set of PowerPoint slides at the bottom, which recommend measuring Lp(a) in the following settings: presence of family history of premature ASCVD and/or increased Lp(a), and all patients with premature or recurrent ASCVD despite LDL-C lowering (Garber et al., 2020).
European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Guidelines for the Management of Dyslipidaemias
The ESC published 2021 guidelines on cardiovascular disease prevention in clinical practice. Their recommendations for CVD risk assessment are included below:
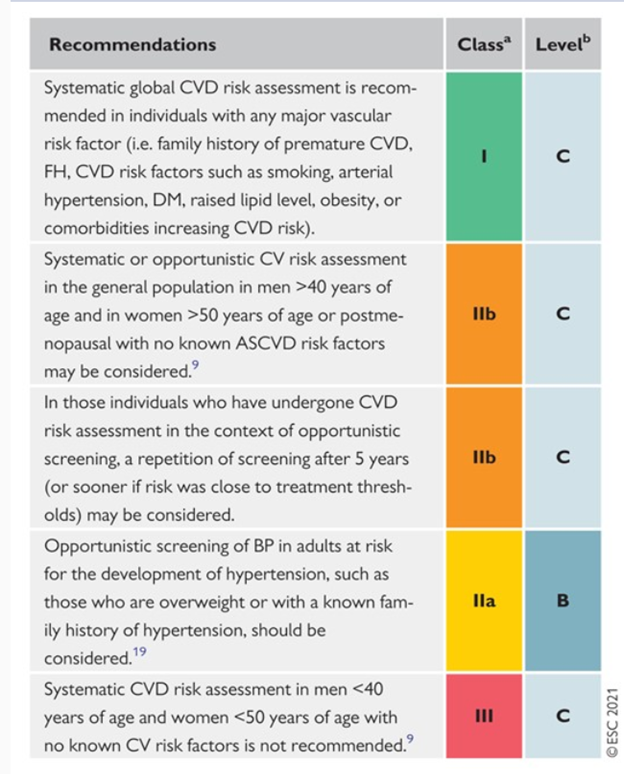
The authors include additional recommendations for CVD risk estimation and modification that are tabulated in the full guideline.
2019 guidelines from the ESC and EAS provided the following recommendations:
- “Lp(a) measurement should be considered at least once in each adult person’s lifetime to identify those with very high inherited Lp(a) levels > 180 mg/dL (> 430 nmol/L) who may have a lifetime risk of ASCVD equivalent to the risk associated with heterozygous familial hypercholesterolemia.
- “Persons with documented ASCVD, type 1 or type 2 DM (T1DM and T2DM, respectively), very high levels of individual risk factors, or chronic kidney disease (CKD) are generally at very-high or high total CV risk. No risk estimation models are needed for such persons …”
- ApoB analysis is recommended for risk assessment, particularly in people with high TG, DM, obesity or metabolic syndrome, or very low LDL-C. It can be used as an alternative to LDL-C, if available, as the primary measurement for screening, diagnosis, and management, and may be preferred over non-HDL-C in people with high TG, DM, obesity, or very low LDL-C.
- CAC score assessment with CT may be helpful in reaching decisions about treatment in people who are at moderate risk of ASCVD. Obtaining such a score may assist in discussions about treatment strategies in patients where the LDL-C goal is not achieved with lifestyle intervention alone and there is a question of whether to institute LDL-C-lowering treatment. Assessment of arterial (carotid or femoral) plaque burden on ultrasonography may also be informative in these circumstances (Mach et al., 2019).”
Total cholesterol may be used to estimate total cardiovascular risk. LDL-C is recommended to be used as the primary lipid analysis for diagnosis, management, screening, and risk estimation. HDL-C and Non-HDL-C are also strong, independent risk factors (Catapano et al., 2016).
Apo B, Lp(a), Apo B/Apo A-I, and Non-HDL-C/HDL-C may all be used as alternative markers for cardiovascular risk. The guidelines note that measuring Apo B and Apo A-I is convenient, accurate, does not require fasting, and is not susceptible to TG levels. The guidelines also recommend against routine measurement of Apo C-III as its use is unknown (Catapano et al., 2016).
In the 2021 ESC Guidelines on cardiovascular disease prevention, the authors stated that “New studies confirm that C-reactive protein has limited additional value. There is renewed interest in lipoprotein(a), but it too provides limited additional value in terms of reclassification potential. Cardiac biomarkers are promising, but further work is needed” (Visseren, Mach, Smulders, Carballo, Koskinas, Bäck, et al., 2021).
European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD)
This joint guideline was published for “diabetes, pre-diabetes, and cardiovascular diseases.” Their specific recommendations relating to cardiovascular risk assessment in patients with diabetes and pre-diabetes are as follows:
- “Routine assessment of microalbuminuria should be carried out to identify patients at risk of developing renal dysfunction and/or CVD.”
- “A resting electrocardiogram (ECG) is indicated in patients with DM and hypertension, or if CVD is suspected.”
- “Other tests, such as transthoracic echocardiography, coronary artery calcium (CAC) score, and ankle-brachial index (ABI), may be considered to test for structural heart disease or as risk modifiers in those at moderate or high risk of CVD.”
- “Routine assessment of novel biomarkers is not recommended for CV risk stratification.”
The guideline noted that “the addition of circulating biomarkers for CV risk assessment has limited clinical value” and stated that “in patients with DM [diabetes mellitus] without known CVD, measurement of C-reactive protein or fibrinogen (inflammatory markers) provides minor incremental value to current risk assessment.” The guideline also noted high-sensitivity cardiac troponin T as not adding incremental “discriminative power” for patients with DM without known CVD (Cosentino et al., 2020), although elevated high-sensitivity cardiac troponin T was noted as an independent predictor of renal decline and CV events in patients with Type 1 diabetes (Cosentino et al., 2020)
Endocrine Society (ES)
This guideline was published with the intent to assess and treat dyslipidemia in patients with endocrine disorders. Their relevant recommendations are listed below:
- “In adults with endocrine disorders, we recommend a lipid panel for the assessment of triglyceride levels and for calculating low-density lipoprotein cholesterol.”
- “In adults with endocrine disorders, we recommend conducting a cardiovascular risk assessment by evaluating traditional risk factors, including the calculation of 10-year atherosclerotic cardiovascular disease risk using a tool such as the Pooled Cohort Equations.”
- “In adults with endocrine disorders at borderline or intermediate risk (10-year atherosclerotic cardiovascular disease risk 5% – 19.9%), particularly those with additional risk-enhancing factors, in whom the decision about statin treatment and/or other preventive interventions is uncertain, we suggest measuring coronary artery calcium to inform shared decision making.”
The guideline also remarks that certain “advanced” lipid testing (assessment of markers such as Apo B, lipid fractionation, and Lp[a]) may be helpful in “characterizing” lipid abnormalities, but “add little” to risk prediction beyond the standard lipid profile.
The guideline goes on to discuss Lp(a), noting that the marker can be helpful in assessing familial risk, but adds “little” in terms of global risk assessment across the general population. The guideline acknowledges other evidence supporting Lp(a)’s use as a marker to manage treatment. Other serum biomarkers and biomarker panels were also considered to add “little” to global risk assessment. Finally, the guideline recommends the use of hs-CRP as a “risk-enhancing factor that may drive more aggressive treatment or the need for advanced risk assessment” (Newman et al., 2020).
Rosenzweig et al. (2019) published on the Primary Prevention of ASCVD and T2DM in Patients at Metabolic Risk. A summary of the recommendations is included below (Rosenzweig et al., 2019):
- “In individuals aged 40 – 75 years in the office setting, we suggest providers screen for all five components of metabolic risk at the clinical visit. The finding of at least three components should specifically alert the clinician to a patient at metabolic risk (at higher risk for atherosclerotic cardiovascular disease and Type 2 diabetes mellitus).
- In individuals aged 40 – 75 years in the office setting who do not yet have atherosclerotic cardiovascular disease or Type 2 diabetes mellitus and already have at least one risk factor, we advise screening every 3 years for all five components of metabolic risk as part of the routine clinical examination.
- To establish metabolic risk in the general population, we recommend that clinicians measure waist circumference as a routine part of the clinical examination.
- In individuals previously diagnosed with prediabetes, we suggest testing at least annually for the presence of overt Type 2 diabetes mellitus.
- We recommend that all individuals at metabolic risk in the office setting have their blood pressure measured annually and, if elevated, at each subsequent visit.
- For individuals with elevated blood pressure above 130 mmHg systolic and/or 80 mmHg diastolic who are not documented as having a history of hypertension, we recommend confirmation of elevated blood pressure on a separate day within a few weeks or with a home blood pressure monitor.”
Components of “metabolic risk” are defined as:
- elevated blood pressure
- increased waist circumference
- elevated fasting triglycerides
- low high-density lipoprotein-cholesterol, and
- elevated glycemia.
American Society for Clinical Pathology
The ASCP recommends against routinely ordering expanded lipid panels (such as particle sizing or nuclear magnetic resonance) as screening for cardiovascular disease (ASCP, 2016).
In 2022, the ASCP published a set of clinical recommendations aiming to provide the guidance and the tools for assessment of ASCVD risk with the goal of appropriately targeting treatment approaches for prevention of ASCVD events, as shown below:
“1
Assessing a patient's risk for ASCVD is the foundation of preventive cardiology and the initial step for determining the appropriateness and intensity of preventive treatment.
2
In primary prevention, global risk scoring is the initial stage for ASCVD risk assessment, providing a calculation of ASCVD risk from a set of standard office-based risk factors for a specified duration (e.g., 10 years) of time, from which a clinician-patient risk discussion is used to discuss the best ways to reduce CVD risk.
3
The presence, quantity, and/or extent of one or more risk enhancing factors, including premature family history, persistently elevated LDL-C, or CKD, as well as severity of certain inflammatory factors such as hsCRP and laboratory measures such as lp(a), can further inform the treatment decision.
4
In women, it is important to take a comprehensive reproductive history from menarche to menopause, including preeclampsia, premature menopause, and autoimmune disease as “risk-enhancing” factors.
5
Race/ethnicity may have a significant impact on the validity of current risk assessment tools and certain higher risk race/ethnic groups may further inform the use of preventive therapy.
6
Social determinants of health may exert independent effects beyond race/ethnicity and need also to be part of the clinician-patient discussion when discussing the most appropriate ways to optimize ASCVD risk.
7
Among subclinical atherosclerotic disease screening tests, CAC is probably the most useful, providing substantial improvement of risk reclassification over global risk scoring in most primary prevention groups, including diabetes. In addition to the consideration of risk enhancing factors (discussed earlier), CAC testing can be used to further inform treatment decisions for preventive therapy, including statin and aspirin use in particular.
8
The use of ABI for assessment of PAD is also valuable and can improve risk reclassification beyond global risk scoring.
9
Carotid ultrasound imaging, if accompanied by carotid plaque assessment may also be useful for risk assessment, especially as an option when CAC scoring is not available.
10
In patients with pre-existing ASCVD, stratification into those at highest risk (e.g., very high risk ASCVD status) for more aggressive treatment is based on the history of multiple major ASCVD events or one major event and multiple high-risk conditions. Moreover, those with recurrent ASCVD events in the short-term define an extreme risk condition warranting even more aggressive risk factor management” (Wong et al., 2022).
Scottish Intercollegiate Guidelines Network
SIGN recommends that patients with established cardiovascular disease, stage 3 chronic kidney disease, micro or macro albuminuria, familial hypercholesterolemia, diabetes and are older than 40 should be considered high risk for cardiovascular events. Diabetes patients under 40 but who have had the disease for 20 years, target organ damage, or other cardiovascular risk factors are also considered high risk.
A cardiovascular risk assessment should be offered to patients 40 or older or any patient with a first degree relative with premature atherosclerotic CVD or familial dyslipidemia, at least every five years.
Asymptomatic individuals should be considered high risk if they are evaluated at a ≥ 20% risk of a first cardiovascular event within ten years.
A lipid profile should include total cholesterol, HDL, triglycerides, and should not be taken during intercurrent illness (SIGN, 2017).
National Institute for Health and Care Excellence (NICE)
A baseline lipid profile should be taken before treatment. This should include total cholesterol, HDL cholesterol, non-HDL, and triglyceride levels. Total and HDL cholesterol should be measured for best estimate of CVD risk.
Omega-3 compounds have “no evidence” to help prevent CVD and NICE recommends against distribution of these compounds for CVD treatment (NICE, 2016).
US Preventive Services Task Force (USPSTF)
The USPSTF Task Force Recommendations include periodic assessment of cardiovascular risk factors from ages 40 to 75 years, including measurement of total cholesterol, LDL-C, and HDL-C levels. The optimal intervals for cardiovascular risk assessment are uncertain. Based on other guidelines and expert opinion, reasonable options include annual assessment of blood pressure and smoking status and measurement of lipid levels every 5 years. Shorter intervals may be useful for persons at higher risk, and longer intervals are appropriate for persons who are regularly at average risk (Bibbins-Domingo et al., 2017).
The USPSTF found insufficient evidence that screening for dyslipidemia in younger adults influences cardiovascular outcomes, and no studies that evaluated the effects of screening vs no screening, treatment vs no treatment, or delayed vs earlier treatment in adults in this age group. Thus, the USPSTF recommends neither for nor against screening for dyslipidemia in this age group. The USPSTF also noted there was insufficient evidence to assess the balance of benefits and harms of screening for dyslipidemia in children and adolescents (Chou et al., 2016).
The USPSTF states that “current evidence is insufficient to assess the benefits and harms of adding ankle-brachial index (ABI), high-sensitivity C-reactive protein (hsCRP) level, or coronary artery calcium (CAC) score to traditional risk assessment for cardiovascular disease (CVD) in asymptomatic adults to prevent CVD events (USPSTF, 2018a).” However, the USPSTF recommends screening for abnormal blood glucose for adults aged 40-70 who are overweight or obese (USPSTF, 2015).
For adults at low risk of CVD events, “The USPSTF recommends against screening with resting or exercise electrocardiography (ECG) to prevent cardiovascular disease (CVD) events” (recommendation grade D, discouraging the use of the service). Moreover, “The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening with resting or exercise ECG to prevent CVD events in asymptomatic adults at intermediate or high risk of CVD events” (recommendation grade I, insufficient evidence) (USPSTF, 2018b).
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for primary hypertension in asymptomatic children and adolescents to prevent subsequent cardiovascular disease (Moyer, 2013). A 2020 recommendation statement by the USPSTF confirmed that the current evidence is insufficient to assess the balance of benefits and harms of screening for high blood pressure in children and adolescents (in general) (USPSTF, 2020). In adults, however, The USPSTF recommends screening for hypertension (persons 18 years or older) with office blood pressure measurement (OBPM). The USPSTF recommends obtaining blood pressure measurements outside of the clinical setting for diagnostic confirmation before starting treatment (USPSTF, 2021).
Finally, screening for obesity in children 6 years or older is recommended (Bibbins-Domingo et al., 2017).
Canadian Cardiovascular Harmonized National Guidelines Endeavour (C-CHANGE)
C-CHANGE recommends a cardiovascular risk assessment for adults from 40 – 75 years old every five years. A risk assessment may also be done when a patient’s expected risk changes.
C-CHANGE recommends plasma lipid screening for adults older than 40 years, as well as lipid screening if a patient has any of the following conditions:
- Clinical evidence of atherosclerosis
- Abdominal aortic aneurysm
- Diabetes mellitus
- Arterial hypertension
- Current cigarette smoking
- Stigmata of dyslipidemia (arcus cornealis xanthelasma or xanthoma)
- Family history of cardiovascular disease‡
- Chronic kidney disease§
- Obesity (BMI ≥ 30 kg/m2)
- Inflammatory disease
- HIV infection
- Erectile dysfunction
- Chronic obstructive pulmonary disease
- Hypertensive diseases of pregnancy
‡Men aged < 55 yr and women aged < 65 yr of age in first-degree relative
§Chronic kidney disease: eGFR < 60 mL/min/1.73 m2 or ACR > 3 mg/mmol for at least 3-mo duration (Tobe et al., 2018)
Canadian Cardiovascular Society (CCS)
In 2021, the CCS published updated their recommendations on the management of dyslipidemia for the prevention of cardiovascular disease in adults. A summary of the society’s recommendations that are relevant to CVD risk assessment is provided below:
- Among women whose pregnancy was complicated by the hypertensive disorders of pregnancy — gestational hypertension and/or preeclampsia — or a preterm birth before 34 weeks’ gestation, a stillbirth and/or a placental abruption, we recommend screening with a comprehensive lipid panel at least 12 weeks postpartum. These women have a higher risk of premature CVD and stroke within 10-15 years after the affected pregnancy (Strong Recommendation, Moderate Quality Evidence).
- To assist with decisions about initiating lipid-lowering pharmacotherapy in a nonpregnant woman who had one or more of these pregnancy complications, we recommend referral to a specialized postpartum cardiovascular health clinic or specialized lipid clinic, if locally available. If such resources are not locally available, we recommend using standard risk assessment tools to decide about lipid-lowering pharmacotherapy. However, when interpreting their 10-year CVD risk using a risk calculator, it is important to note that most women in this group will be found to have a low calculated absolute risk of CVD, short-term, which may give a false sense of reassurance to both the patient and her health care provider. (Weak Recommendation; Low-Quality Evidence).
- For any patient with triglycerides > 1.5mM, use non-HDL-C or apoB instead of LDL-C as the preferred lipid parameter for initial screening and treatment target (< 2.6 mM for non-HDL-C or < 0.8 g/L for apoB) in intermediate or high risk individuals (Strong Recommendation, High-Quality Evidence).
- We recommend measuring lipoprotein (a) level once in a person’s lifetime as a part of the initial lipid screening (Strong Recommendation; High-Quality Evidence).
- We suggest that CAC [coronary artery calcium] screening using computed tomography imaging might be considered for asymptomatic adults 40 years or older and at intermediate risk (FRS 10% – 20%) for whom treatment decisions are uncertain (Strong Recommendation, Moderate-Quality Evidence).
- We recommend that CAC screening using computed tomography imaging not be undertaken for: (1) high-risk individuals; (2) patients receiving statin treatment; or (3) most asymptomatic, low-risk adults (Strong Recommendation; Moderate-Quality Evidence).
- We suggest that CAC screening might be considered for a subset of low-risk individuals 40 years or older (Weak Recommendation; Low-Quality Evidence).” (Pearson et al., 2021)
The 2021 guidelines affirmed those from 2016, stating that “Screening should be repeated every 5 years for men and women aged 40 – 75 years using the modified FRS or Cardiovascular Life Expectancy Model (CLEM) to guide therapy to reduce major CV events.”
A revision of the 2016 recommendation is the role of Lp(a): “Lp(a) is not currently considered a treatment target and repeat measures are therefore not indicated.” Moreover, non-fasting lipid testing is recommended during the CV risk assessment, and “It is now generally preferable to follow non-HDL-C or ApoB levels over LDL-C when interpreting lipid results, particularly when TG are ≥ 1.5 mmol/L” (Pearson et al., 2021)
HIV Medicine Association of the Infectious Diseases Society of America
HIV-infected patients commonly develop dyslipidemia after starting antiretroviral therapy (ART). The lipid abnormalities developed in HIV-infected patients are associated with increased cardiovascular risk. HIV Medicine Association of the Infectious Diseases Society of America have updated their guidelines in 2013 to include a new section on metabolic comorbidities. They recommend obtaining a fasting lipid profile prior to and within 1 – 3 months after starting ART and every 6 – 12 months in all patients. The 2020 update affirms this, as under the workup for routine healthcare maintenance considerations for persons with HIV, they recommend the following steps: “Lipid profile: perform every 5 years if normal; more frequently if abnormal or other cardiovascular risk factors present (every 6 – 12 months); if abnormal, repeat fasting”, and asks that clinicians “Follow the atherosclerotic cardiovascular disease risk calculator. Consider testing 1–3 months after starting or changing ART” (Thompson et al., 2020).
The Association also notes that HbA1C may be tested or used for screening and states that a lower cutoff of 5.8% for diabetes may be used for patients on ART instead of the higher 6.5%. Finally, the Association recommends measuring HbA1C every six months in patients with diabetes (Aberg et al., 2014; Thompson et al., 2020).
According to the Association, an initial evaluation and an immediate follow-up for persons with HIV includes “A comprehensive present and past medical history that includes HIV-related information, medication/social/family history …, review of systems, and physical examination … should be obtained for all patients upon initiation of care, ideally at the first visit or, if not feasible, as soon as possible thereafter,” and this includes testing for “cardiovascular disease and risk factors, including hyperlipidemia, hypertension, diabetes mellitus, smoking” (Thompson et al., 2020).
U.S. Department of Veterans Affairs (VA) and U.S. Department of Defense (DoD)
The United States VA and DoD published a joint guideline regarding management of dyslipidemia for reducing CVD risk in adults. Their relevant recommendations are listed below:
- “For primary prevention in patients over age 40 and not on statin therapy who have not developed new cardiovascular risk factors (e.g., diabetes, hypertension, tobacco use), we suggest against offering a cardiovascular disease risk assessment more frequently than every five years.”
- “For primary prevention in patients not on statin therapy, we suggest against routinely ordering a lipid panel more frequently than every 10 years.”
- “For cardiovascular risk assessment in primary prevention, we suggest using a 10-year risk calculator.”
- “We suggest against the routine use of coronary artery calcium testing.”
- “We suggest against the routine use of additional risk markers (e.g., high-sensitivity C-reactive protein, ankle-brachial index, coronary artery calcium) when assessing cardiovascular risk.”
The guideline also remarks that several other markers, such as “coronary artery calcium (CAC), high-sensitivity C-reactive protein, ankle–brachial index, and apolipoprotein evaluations” have been proposed as useful tools to determine risk. However, these markers have been deemed “limited in further refining risk”. Although CAC was considered to best of the markers listed, the guideline still recommended against routine CAC testing.
The guideline also recommends against “routine lipid level testing for risk assessment and monitoring, unless it is specifically intended to guide decision making” (O'Malley et al., 2020).
2016 European Society of Cardiology (ESC) and Other Societies on Cardiovascular Disease Prevention in Clinical Practice
In 2016, the Sixth Joint Task Force of the ESC and Other Societies on Cardiovascular Disease Prevention in Clinical Practice published guidelines on cardiovascular disease prevention in clinical practice.
The authors state that routine assessment of circulating or urinary biomarkers is not recommended for CVD risk stratification. The Task Force states that there is conflicting data on the utility of these biomarkers (such as hsCRP, various apolipoproteins, etc.) and that the overall contributions of these biomarkers to the current methods of risk assessment are minor. Furthermore, the degree to which these biomarkers do influence a patient’s total CVD risk is highly variable (Piepoli et al., 2016).
The Task Force recommends repeating risk assessment every 5 years, and more often for higher risk patients. However, the Task Force only recommends this screening procedure for men > 40 years and women > 50 years or post-menopausal, declaring that “systematic risk assessment in men < 40 years and women < 50 with no known CV risk factors is not recommended” due to low cost effectiveness (Piepoli et al., 2016).
References
- 360dx. (2019). Immunodiagnostics Firm Singulex Shutters Abruptly. Retrieved 1/4/2021 from https://www.360dx.com/immunoassays/immunodiagnostics-firm-singulex-shutters-abruptly#.X_M7DFxKiUk
- AACE. (2021). CONSENSUS STATEMENT BY THE AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY ON THE MANAGEMENT OF DYSLIPIDEMIA AND PREVENTION OF CARDIOVASCULAR DISEASE ALGORITHM – 2020 EXECUTIVE SUMMARY. https://pro.aace.com/pdfs/lipids/CS-2020-0490.pdf
- Aberg, J. A., Gallant, J. E., Ghanem, K. G., Emmanuel, P., Zingman, B. S., & Horberg, M. A. (2014). Primary Care Guidelines for the Management of Persons Infected With HIV: 2013 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical Infectious Diseases, 58(1), e1-e34. https://doi.org/10.1093/cid/cit665
- ACC. (2018). 2018 Guideline on the Management of Blood Cholesterol. https://www.acc.org/~/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Guidelines/2018/Guidelines-Made-Simple-Tool-2018-Cholesterol.pdf
- ADA. (2020). Standards of Medical Care in diabetes—2020. https://care.diabetesjournals.org/content/43/Supplement_1
- ADA. (2021a). Standards of Medical Care in diabetes—2021, Chapter 10. Retrieved 12/30/2020 from https://care.diabetesjournals.org/content/44/Supplement_1/S125
- ADA. (2021b). Standards of Medical Care in diabetes—2021, Chapter 13. Retrieved 12/30/2020 from https://care.diabetesjournals.org/content/44/Supplement_1/S180
- Akcay, M., & Yuksel, S. (2019). Isotretinoin-associated possible Kounis syndrome: A case report and a review of other cardiovascular side effects reported in the literature. Turk Kardiyol Dern Ars, 47(4), 324-328. https://doi.org/10.5543/tkda.2018.67055 (Isotretinoin ile iliskili olasi Kounis sendromu: Olgu sunumu ve diger kardiyovaskuler yan etkilerin literatur derlemesi.)
- Alan, S., Unal, B., & Yildirim, A. (2016). Premature ventricular contractions associated with isotretinoin use. An Bras Dermatol, 91(6), 820-821. https://doi.org/10.1590/abd1806-4841.20165138
- Antonopoulos, A. S., Angelopoulos, A., Papanikolaou, P., Simantiris, S., Oikonomou, E. K., Vamvakaris, K., Koumpoura, A., Farmaki, M., Trivella, M., Vlachopoulos, C., Tsioufis, K., Antoniades, C., & Tousoulis, D. (2022). Biomarkers of Vascular Inflammation for Cardiovascular Risk Prognostication: A Meta-Analysis. JACC Cardiovasc Imaging, 15(3), 460-471. https://doi.org/10.1016/j.jcmg.2021.09.014
- Arnett, D. K., Blumenthal, R. S., Albert, M. A., Buroker, A. B., Goldberger, Z. D., Hahn, E. J., Himmelfarb, C. D., Khera, A., Lloyd-Jones, D., McEvoy, J. W., Michos, E. D., Miedema, M. D., Munoz, D., Smith, S. C., Jr., Virani, S. S., Williams, K. A., Sr., Yeboah, J., & Ziaeian, B. (2019). 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 140(11), e596-e646. https://doi.org/10.1161/cir.0000000000000678
- Arsenault, B. J., Boekholdt, S. M., Hovingh, G. K., Hyde, C. L., DeMicco, D. A., Chatterjee, A., Barter, P., Deedwania, P., Waters, D. D., LaRosa, J. C., Pedersen, T. R., & Kastelein, J. J. (2012). The 719Arg variant of KIF6 and cardiovascular outcomes in statin-treated, stable coronary patients of the treating to new targets and incremental decrease in end points through aggressive lipid-lowering prospective studies. Circ Cardiovasc Genet, 5(1), 51-57. https://doi.org/10.1161/circgenetics.111.960252
- ASCP. (2016). American Society for Clinical Pathology. http://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease/
- Ashley, E. A., Hershberger, R. E., Caleshu, C., Ellinor, P. T., Garcia, J. G., Herrington, D. M., Ho, C. Y., Johnson, J. A., Kittner, S. J., Macrae, C. A., Mudd-Martin, G., Rader, D. J., Roden, D. M., Scholes, D., Sellke, F. W., Towbin, J. A., Van Eyk, J., & Worrall, B. B. (2012). Genetics and cardiovascular disease: a policy statement from the American Heart Association. Circulation, 126(1), 142-157. https://doi.org/10.1161/CIR.0b013e31825b07f8
- Beauchemin, M., Geguchadze, R., Guntur, A. R., Nevola, K., Le, P. T., Barlow, D., Rue, M., Vary, C. P. H., Lary, C. W., Motyl, K. J., & Houseknecht, K. L. (2019). Exploring mechanisms of increased cardiovascular disease risk with antipsychotic medications: Risperidone alters the cardiac proteomic signature in mice. Pharmacol Res, 152, 104589. https://doi.org/10.1016/j.phrs.2019.104589
- Bibbins-Domingo, K., University of California, S. F., Grossman, D. C., Group Health Research Institute, S., Washington, Curry, S. J., University of Iowa, I. C., Davidson, K. W., Columbia University, N. Y., New York, Epling, J. W., State University of New York Upstate Medical University, S., García, F. A. R., Pima County Department of Health, T., Arizona, Gillman, M. W., Harvard Medical School and Harvard Pilgrim Health Care Institute, B., Massachusetts, Now with the National Institutes of Health, B., Maryland (was not affiliated with the National Institutes of Health while a member of the USPSTF), Kemper, A. R., Duke University, D., North Carolina, Krist, A. H., Fairfax Family Practice Residency, F., Virginia, Virginia Commonwealth University, R., Kurth, A. E., Yale University, N. H., Connecticut, Landefeld, C. S., Birmingham, U. o. A. a., LeFevre, M. L., University of Missouri, C., Mangione, C. M., University of California, L. A., Phillips, W. R., University of Washington, S., Owens, D. K., Stanford University, S., California, Phipps, M. G., Brown University, P., Rhode Island, Pignone, M. P., & Austin, U. o. T. a. (2017). Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. Jama, 316(19), 1997-2007. https://doi.org/10.1001/jama.2016.15450
- Boekholdt, S. M., Hovingh, G. K., Mora, S., Arsenault, B. J., Amarenco, P., Pedersen, T. R., LaRosa, J. C., Waters, D. D., DeMicco, D. A., Simes, R. J., Keech, A. C., Colquhoun, D., Hitman, G. A., Betteridge, D. J., Clearfield, M. B., Downs, J. R., Colhoun, H. M., Gotto, A. M., Jr., Ridker, P. M., Grundy, S. M., & Kastelein, J. J. (2014). Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol, 64(5), 485-494. https://doi.org/10.1016/j.jacc.2014.02.615
- Bosch, J., Gerstein, H. C., Dagenais, G. R., Diaz, R., Dyal, L., Jung, H., Maggiono, A. P., Probstfield, J., Ramachandran, A., Riddle, M. C., Ryden, L. E., & Yusuf, S. (2012). n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med, 367(4), 309-318. https://doi.org/10.1056/NEJMoa1203859
- Cao, J., Nomura, S. O., Steffen, B. T., Guan, W., Remaley, A. T., Karger, A. B., Ouyang, P., Michos, E. D., & Tsai, M. Y. (2019). Apolipoprotein B discordance with low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol in relation to coronary artery calcification in the Multi-Ethnic Study of Atherosclerosis (MESA). J Clin Lipidol. https://doi.org/10.1016/j.jacl.2019.11.005
- CardioDX. (2019). Corus CAD Overview. http://www.cardiodx.com/patient-resources/corus-cad-test/
- Catapano, A. L., Graham, I., De Backer, G., Wiklund, O., Chapman, M. J., Drexel, H., Hoes, A. W., Jennings, C. S., Landmesser, U., Pedersen, T. R., Reiner, Z., Riccardi, G., Taskinen, M. R., Tokgozoglu, L., Verschuren, W. M. M., Vlachopoulos, C., Wood, D. A., Zamorano, J. L., & Cooney, M. T. (2016). 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J, 37(39), 2999-3058. https://doi.org/10.1093/eurheartj/ehw272
- CDC. (2017a). Cardiovascular Disease Biomarker Standardization Programs. https://www.cdc.gov/labstandards/cvd.html
- CDC. (2017b). LSP: Lipids Standardization Program. https://www.cdc.gov/labstandards/lsp.html
- CDC. (2019). Know Your Risk for Heart Disease. https://www.cdc.gov/heartdisease/risk_factors.htm
- Charland, S. L., Agatep, B. C., Herrera, V., Schrader, B., Frueh, F. W., Ryvkin, M., Shabbeer, J., Devlin, J. J., Superko, H. R., & Stanek, E. J. (2014). Providing patients with pharmacogenetic test results affects adherence to statin therapy: results of the Additional KIF6 Risk Offers Better Adherence to Statins (AKROBATS) trial. Pharmacogenomics J, 14(3), 272-280. https://doi.org/10.1038/tpj.2013.27
- Chiesa, S. T., Charakida, M., Georgiopoulos, G., Roberts, J. D., Stafford, S. J., Park, C., Mykkänen, J., Kähönen, M., Lehtimäki, T., Ala-Korpela, M., Raitakari, O., Pietiäinen, M., Pussinen, P., Muthurangu, V., Hughes, A. D., Sattar, N., Timpson, N. J., & Deanfield, J. E. (2022). Glycoprotein Acetyls: A Novel Inflammatory Biomarker of Early Cardiovascular Risk in the Young. J Am Heart Assoc, 11(4), e024380. https://doi.org/10.1161/jaha.121.024380
- Chou, R., Dana, T., Blazina, I., Daeges, M., Bougatsos, C., & Jeanne, T. L. (2016). Screening for Dyslipidemia in Younger Adults: A Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med, 165(8), 560-564. https://doi.org/10.7326/m16-0946
- Colucci, W., & Chen, H. H. (2022, 4/26/2022). Natriuretic peptide measurement in heart failure. Retrieved 5/11/2022 from https://www.uptodate.com/contents/natriuretic-peptide-measurement-in-heart-failure?search=Brain%20natriuretic%20peptide&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Cosentino, F., Grant, P. J., Aboyans, V., Bailey, C. J., Ceriello, A., Delgado, V., Federici, M., Filippatos, G., Grobbee, D. E., Hansen, T. B., Huikuri, H. V., Johansson, I., Jüni, P., Lettino, M., Marx, N., Mellbin, L. G., Östgren, C. J., Rocca, B., Roffi, M., Sattar, N., Seferović, P. M., Sousa-Uva, M., Valensi, P., Wheeler, D. C., & Group, E. S. C. S. D. (2020). 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). European Heart Journal, 41(2), 255-323. https://doi.org/10.1093/eurheartj/ehz486
- Crea, F., Morrow, David. (2020, 8/20/2022). C-reactive protein in cardiovascular disease. Retrieved 5/11/2022 from https://www.uptodate.com/contents/c-reactive-protein-in-cardiovascular-disease?search=screening-for-cardiovascular-risk-with-c-reactive-protein&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- de Oliveira Otto, M. C., Wu, J. H., Baylin, A., Vaidya, D., Rich, S. S., Tsai, M. Y., Jacobs, D. R., Jr., & Mozaffarian, D. (2013). Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc, 2(6), e000506. https://doi.org/10.1161/jaha.113.000506
- De Stefano, A., Mannucci, L., Tamburi, F., Cardillo, C., Schinzari, F., Rovella, V., Nistico, S., Bennardo, L., Di Daniele, N., & Tesauro, M. (2019). Lp-PLA2, a new biomarker of vascular disorders in metabolic diseases. Int J Immunopathol Pharmacol, 33, 2058738419827154. https://doi.org/10.1177/2058738419827154
- Dehghan, A., Bis, J. C., White, C. C., Smith, A. V., Morrison, A. C., Cupples, L. A., Trompet, S., Chasman, D. I., Lumley, T., Völker, U., Buckley, B. M., Ding, J., Jensen, M. K., Folsom, A. R., Kritchevsky, S. B., Girman, C. J., Ford, I., Dörr, M., Salomaa, V., Uitterlinden, A. G., Eiriksdottir, G., Vasan, R. S., Franceschini, N., Carty, C. L., Virtamo, J., Demissie, S., Amouyel, P., Arveiler, D., Heckbert, S. R., Ferrières, J., Ducimetière, P., Smith, N. L., Wang, Y. A., Siscovick, D. S., Rice, K. M., Wiklund, P.-G., Taylor, K. D., Evans, A., Kee, F., Rotter, J. I., Karvanen, J., Kuulasmaa, K., Heiss, G., Kraft, P., Launer, L. J., Hofman, A., Markus, M. R. P., Rose, L. M., Silander, K., Wagner, P., Benjamin, E. J., Lohman, K., Stott, D. J., Rivadeneira, F., Harris, T. B., Levy, D., Liu, Y., Rimm, E. B., Jukema, J. W., Völzke, H., Ridker, P. M., Blankenberg, S., Franco, O. H., Gudnason, V., Psaty, B. M., Boerwinkle, E., & O'Donnell, C. J. (2016). Genome-Wide Association Study for Incident Myocardial Infarction and Coronary Heart Disease in Prospective Cohort Studies: The CHARGE Consortium. PLoS One, 11(3), e0144997. https://doi.org/10.1371/journal.pone.0144997
- Deloukas, P., Kanoni, S., Willenborg, C., Farrall, M., Assimes, T. L., Thompson, J. R., Ingelsson, E., Saleheen, D., Erdmann, J., Goldstein, B. A., Stirrups, K., Konig, I. R., Cazier, J. B., Johansson, A., Hall, A. S., Lee, J. Y., Willer, C. J., Chambers, J. C., Esko, T., Folkersen, L., Goel, A., Grundberg, E., Havulinna, A. S., Ho, W. K., Hopewell, J. C., Eriksson, N., Kleber, M. E., Kristiansson, K., Lundmark, P., Lyytikainen, L. P., Rafelt, S., Shungin, D., Strawbridge, R. J., Thorleifsson, G., Tikkanen, E., Van Zuydam, N., Voight, B. F., Waite, L. L., Zhang, W., Ziegler, A., Absher, D., Altshuler, D., Balmforth, A. J., Barroso, I., Braund, P. S., Burgdorf, C., Claudi-Boehm, S., Cox, D., Dimitriou, M., Do, R., Doney, A. S., El Mokhtari, N., Eriksson, P., Fischer, K., Fontanillas, P., Franco-Cereceda, A., Gigante, B., Groop, L., Gustafsson, S., Hager, J., Hallmans, G., Han, B. G., Hunt, S. E., Kang, H. M., Illig, T., Kessler, T., Knowles, J. W., Kolovou, G., Kuusisto, J., Langenberg, C., Langford, C., Leander, K., Lokki, M. L., Lundmark, A., McCarthy, M. I., Meisinger, C., Melander, O., Mihailov, E., Maouche, S., Morris, A. D., Muller-Nurasyid, M., Nikus, K., Peden, J. F., Rayner, N. W., Rasheed, A., Rosinger, S., Rubin, D., Rumpf, M. P., Schafer, A., Sivananthan, M., Song, C., Stewart, A. F., Tan, S. T., Thorgeirsson, G., van der Schoot, C. E., Wagner, P. J., Wells, G. A., Wild, P. S., Yang, T. P., Amouyel, P., Arveiler, D., Basart, H., Boehnke, M., Boerwinkle, E., Brambilla, P., Cambien, F., Cupples, A. L., de Faire, U., Dehghan, A., Diemert, P., Epstein, S. E., Evans, A., Ferrario, M. M., Ferrieres, J., Gauguier, D., Go, A. S., Goodall, A. H., Gudnason, V., Hazen, S. L., Holm, H., Iribarren, C., Jang, Y., Kahonen, M., Kee, F., Kim, H. S., Klopp, N., Koenig, W., Kratzer, W., Kuulasmaa, K., Laakso, M., Laaksonen, R., Lind, L., Ouwehand, W. H., Parish, S., Park, J. E., Pedersen, N. L., Peters, A., Quertermous, T., Rader, D. J., Salomaa, V., Schadt, E., Shah, S. H., Sinisalo, J., Stark, K., Stefansson, K., Tregouet, D. A., Virtamo, J., Wallentin, L., Wareham, N., Zimmermann, M. E., Nieminen, M. S., Hengstenberg, C., Sandhu, M. S., Pastinen, T., Syvanen, A. C., Hovingh, G. K., Dedoussis, G., Franks, P. W., Lehtimaki, T., Metspalu, A., Zalloua, P. A., Siegbahn, A., Schreiber, S., Ripatti, S., Blankenberg, S. S., Perola, M., Clarke, R., Boehm, B. O., O'Donnell, C., Reilly, M. P., Marz, W., Collins, R., Kathiresan, S., Hamsten, A., Kooner, J. S., Thorsteinsdottir, U., Danesh, J., Palmer, C. N., Roberts, R., Watkins, H., Schunkert, H., & Samani, N. J. (2013). Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet, 45(1), 25-33. https://doi.org/10.1038/ng.2480
- Di Angelantonio, E., Sarwar, N., Perry, P., Kaptoge, S., Ray, K. K., Thompson, A., Wood, A. M., Lewington, S., Sattar, N., Packard, C. J., Collins, R., Thompson, S. G., & Danesh, J. (2009). Major lipids, apolipoproteins, and risk of vascular disease. Jama, 302(18), 1993-2000. https://doi.org/10.1001/jama.2009.1619
- Dutta, A., Henley, W., Lang, I. A., Murray, A., Guralnik, J., Wallace, R. B., & Melzer, D. (2011). The coronary artery disease-associated 9p21 variant and later life 20-year survival to cohort extinction. Circ Cardiovasc Genet, 4(5), 542-548. https://doi.org/10.1161/circgenetics.111.960146
- Elashoff, M. R., Wingrove, J. A., Beineke, P., Daniels, S. E., Tingley, W. G., Rosenberg, S., Voros, S., Kraus, W. E., Ginsburg, G. S., Schwartz, R. S., Ellis, S. G., Tahirkheli, N., Waksman, R., McPherson, J., Lansky, A. J., & Topol, E. J. (2011). Development of a blood-based gene expression algorithm for assessment of obstructive coronary artery disease in non-diabetic patients. BMC Med Genomics, 4, 26. https://doi.org/10.1186/1755-8794-4-26
- Ford, I., Shah, A. S., Zhang, R., McAllister, D. A., Strachan, F. E., Caslake, M., Newby, D. E., Packard, C. J., & Mills, N. L. (2016). High-Sensitivity Cardiac Troponin, Statin Therapy, and Risk of Coronary Heart Disease. J Am Coll Cardiol, 68(25), 2719-2728. https://doi.org/10.1016/j.jacc.2016.10.020
- Ganesh, S. K., Arnett, D. K., Assimes, T. L., Basson, C. T., Chakravarti, A., Ellinor, P. T., Engler, M. B., Goldmuntz, E., Herrington, D. M., Hershberger, R. E., Hong, Y., Johnson, J. A., Kittner, S. J., McDermott, D. A., Meschia, J. F., Mestroni, L., O'Donnell, C. J., Psaty, B. M., Vasan, R. S., Ruel, M., Shen, W. K., Terzic, A., & Waldman, S. A. (2013). Genetics and genomics for the prevention and treatment of cardiovascular disease: update: a scientific statement from the American Heart Association. Circulation, 128(25), 2813-2851. https://doi.org/10.1161/01.cir.0000437913.98912.1d
- Garber, A. J., Handelsman, Y., Grunberger, G., Einhorn, D., Abrahamson, M. J., Barzilay, J. I., Blonde, L., Bush, M. A., DeFronzo, R. A., Garber, J. R., Garvey, W. T., Hirsch, I. B., Jellinger, P. S., McGill, J. B., Mechanick, J. I., Perreault, L., Rosenblit, P. D., Samson, S., & Umpierrez, G. E. (2020). CONSENSUS STATEMENT BY THE AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY ON THE COMPREHENSIVE TYPE 2 DIABETES MANAGEMENT ALGORITHM – 2020 EXECUTIVE SUMMARY. Endocrine Practice, 26(1), 107-139. https://doi.org/10.4158/CS-2019-0472
- Garg, P. K., McClelland, R. L., Jenny, N. S., Criqui, M. H., Greenland, P., Rosenson, R. S., Siscovick, D. S., Jorgensen, N., & Cushman, M. (2015). Lipoprotein-associated phospholipase A2 and risk of incident cardiovascular disease in a multi-ethnic cohort: The multi ethnic study of atherosclerosis. Atherosclerosis, 241(1), 176-182. https://doi.org/10.1016/j.atherosclerosis.2015.05.006
- Genova. (2021). CV Health Plus Genomics™. https://www.gdx.net/uk/core-uk/one-page-test-descriptions-uk/CVHealth-Genomics-Test-Description-MET14.pdf
- Gibson, M., Morrow, D. (2022, 4/5/2022). Elevated cardiac troponin concentration in the absence of an acute coronary syndrome. Retrieved 5/11/2022 from https://www.uptodate.com/contents/elevated-cardiac-troponin-concentration-in-the-absence-of-an-acute-coronary-syndrome?search=troponin&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2
- Goff, D. C., Jr., Lloyd-Jones, D. M., Bennett, G., Coady, S., D'Agostino, R. B., Sr., Gibbons, R., Greenland, P., Lackland, D. T., Levy, D., O'Donnell, C. J., Robinson, J. G., Schwartz, J. S., Shero, S. T., Smith, S. C., Jr., Sorlie, P., Stone, N. J., & Wilson, P. W. (2014). 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 63(25 Pt B), 2935-2959. https://doi.org/10.1016/j.jacc.2013.11.005
- Gransbo, K., Almgren, P., Sjogren, M., Smith, J. G., Engstrom, G., Hedblad, B., & Melander, O. (2013). Chromosome 9p21 genetic variation explains 13% of cardiovascular disease incidence but does not improve risk prediction. J Intern Med, 274(3), 233-240. https://doi.org/10.1111/joim.12063
- Greenland, P., Alpert, J. S., Beller, G. A., Benjamin, E. J., Budoff, M. J., Fayad, Z. A., Foster, E., Hlatky, M. A., Hodgson, J. M., Kushner, F. G., Lauer, M. S., Shaw, L. J., Smith, S. C., Jr., Taylor, A. J., Weintraub, W. S., Wenger, N. K., Jacobs, A. K., Anderson, J. L., Albert, N., Buller, C. E., Creager, M. A., Ettinger, S. M., Guyton, R. A., Halperin, J. L., Hochman, J. S., Nishimura, R., Ohman, E. M., Page, R. L., Stevenson, W. G., Tarkington, L. G., & Yancy, C. W. (2010). 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 56(25), e50-103. https://doi.org/10.1016/j.jacc.2010.09.001
- Grundy, S. M., Stone Neil, J., Bailey Alison, L., Beam, C., Birtcher Kim, K., Blumenthal Roger, S., Braun Lynne, T., de Ferranti, S., Faiella-Tommasino, J., Forman Daniel, E., Goldberg, R., Heidenreich Paul, A., Hlatky Mark, A., Jones Daniel, W., Lloyd-Jones, D., Lopez-Pajares, N., Ndumele Chiadi, E., Orringer Carl, E., Peralta Carmen, A., Saseen Joseph, J., Smith Sidney, C., Sperling, L., Virani Salim, S., & Yeboah, J. (2019). 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 139(25), e1082-e1143. https://doi.org/10.1161/CIR.0000000000000625
- Guler, E., Babur Guler, G., Yavuz, C., & Kizilirmak, F. (2015). An unknown side effect of isotretinoin: pericardial effusion with atrial tachycardia. Anatol J Cardiol, 15(2), 168-169. https://doi.org/10.5152/akd.2015.5790
- HeartLab, C. (2020). Retrieved 1/4/2021 from http://www.clevelandheartlab.com/providers/the-science/
- Hopewell, J. C., Parish, S., Clarke, R., Armitage, J., Bowman, L., Hager, J., Lathrop, M., & Collins, R. (2011). No impact of KIF6 genotype on vascular risk and statin response among 18,348 randomized patients in the heart protection study. J Am Coll Cardiol, 57(20), 2000-2007. https://doi.org/10.1016/j.jacc.2011.02.015
- Howell, S., Yarovova, E., Khwanda, A., & Rosen, S. D. (2019). Cardiovascular effects of psychotic illnesses and antipsychotic therapy. Heart, 105(24), 1852-1859. https://doi.org/10.1136/heartjnl-2017-312107
- Hwang, Y. C., Ahn, H. Y., Han, K. H., Park, S. W., & Park, C. Y. (2017). Prediction of future cardiovascular disease with an equation to estimate apolipoprotein B in patients with high cardiovascular risk: an analysis from the TNT and IDEAL study. Lipids Health Dis, 16(1), 158. https://doi.org/10.1186/s12944-017-0549-8
- Iakoubova, O. A., Tong, C. H., Rowland, C. M., Kirchgessner, T. G., Young, B. A., Arellano, A. R., Shiffman, D., Sabatine, M. S., Campos, H., Packard, C. J., Pfeffer, M. A., White, T. J., Braunwald, E., Shepherd, J., Devlin, J. J., & Sacks, F. M. (2008). Association of the Trp719Arg polymorphism in kinesin-like protein 6 with myocardial infarction and coronary heart disease in 2 prospective trials: the CARE and WOSCOPS trials. J Am Coll Cardiol, 51(4), 435-443. https://doi.org/10.1016/j.jacc.2007.05.057
- Itakura, H., Yokoyama, M., Matsuzaki, M., Saito, Y., Origasa, H., Ishikawa, Y., Oikawa, S., Sasaki, J., Hishida, H., Kita, T., Kitabatake, A., Nakaya, N., Sakata, T., Shimada, K., Shirato, K., & Matsuzawa, Y. (2011). Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb, 18(2), 99-107. http://dx.doi.org/
- Jaffe, A. (2022, 3/1/2022). Troponin testing: Analytical considerations. Retrieved 5/11/2022 from https://www.uptodate.com/contents/troponin-testing-analytical-considerations?topicRef=86&source=see_link
- Jaffe, A., Morrow, David. (2021, 2/15/2021). Biomarkers of cardiac injury other than troponin. Retrieved 5/11/2022 from https://www.uptodate.com/contents/biomarkers-of-cardiac-injury-other-than-troponin?sectionName=Why%20troponin%20is%20preferred&topicRef=86&anchor=H516921337&source=see_link#H516921337
- Januzzi, J. L., Jr., Ahmad, T., Mulder, H., Coles, A., Anstrom, K. J., Adams, K. F., Ezekowitz, J. A., Fiuzat, M., Houston-Miller, N., Mark, D. B., Piña, I. L., Passmore, G., Whellan, D. J., Cooper, L. S., Leifer, E. S., Desvigne-Nickens, P., Felker, G. M., & O'Connor, C. M. (2019). Natriuretic Peptide Response and Outcomes in Chronic Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol, 74(9), 1205-1217. https://doi.org/10.1016/j.jacc.2019.06.055
- Jellinger, P. S., Handelsman, Y., Rosenblit, P. D., Bloomgarden, Z. T., Fonseca, V. A., Garber, A. J., Grunberger, G., Guerin, C. K., Bell, D. S. H., Mechanick, J. I., Pessah-Pollack, R., Wyne, K., Smith, D., Brinton, E. A., Fazio, S., & Davidson, M. (2017). AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY GUIDELINES FOR MANAGEMENT OF DYSLIPIDEMIA AND PREVENTION OF CARDIOVASCULAR DISEASE. Endocr Pract, 23(Suppl 2), 1-87. https://doi.org/10.4158/ep171764.appgl
- Jepsen, A.-M. K., Langsted, A., Varbo, A., Bang, L. E., Kamstrup, P. R., & Nordestgaard, B. G. (2016). Increased Remnant Cholesterol Explains Part of Residual Risk of All-Cause Mortality in 5414 Patients with Ischemic Heart Disease. Clinical Chemistry, 62(4), 593. https://doi.org/10.1373/clinchem.2015.253757
- Joshi, P. H., Khokhar, A. A., Massaro, J. M., Lirette, S. T., Griswold, M. E., Martin, S. S., Blaha, M. J., Kulkarni, K. R., Correa, A., Ralph B. D'Agostino, S., Jones, S. R., Toth, P. P., & Group, t. L. I. C. L. S. (2016). Remnant Lipoprotein Cholesterol and Incident Coronary Heart Disease: The Jackson Heart and Framingham Offspring Cohort Studies. https://doi.org/10.1161/JAHA.115.002765
- Karadag, A. S., Gumrukcuoglu, H. A., Gunes Bilgili, S., Ozkol, H. U., Ertugrul, D. T., Simsek, H., Sahin, M., & Calka, O. (2012). Does isotretinoin therapy have any effects on electrocardiography, heart rate and blood pressure? J Dermatolog Treat, 23(3), 168-171. https://doi.org/10.3109/09546634.2010.546831
- Kessler, T., Vilne, B., & Schunkert, H. (2016). The impact of genome-wide association studies on the pathophysiology and therapy of cardiovascular disease. EMBO Mol Med, 8(7), 688-701. https://doi.org/10.15252/emmm.201506174
- Kilicaslan, E. E., Karakilic, M., & Erol, A. (2019). The Relationship between 10 Years Risk of Cardiovascular Disease and Schizophrenia Symptoms: Preliminary Results. Psychiatry Investig, 16(12), 933-939. https://doi.org/10.30773/pi.2019.0063
- Kongpakwattana, K., Ademi, Z., Chaiyasothi, T., Nathisuwan, S., Zomer, E., Liew, D., & Chaiyakunapruk, N. (2019). Cost-Effectiveness Analysis of Non-Statin Lipid-Modifying Agents for Secondary Cardiovascular Disease Prevention Among Statin-Treated Patients in Thailand. Pharmacoeconomics, 37(10), 1277-1286. https://doi.org/10.1007/s40273-019-00820-6
- Kuwahara, K., Nakagawa, Y., & Nishikimi, T. (2018). Cutting Edge of Brain Natriuretic Peptide (BNP) Research - The Diversity of BNP Immunoreactivity and Its Clinical Relevance. Circ J, 82(10), 2455-2461. https://doi.org/10.1253/circj.CJ-18-0824
- Lamprea-Montealegre, J. A., Staplin, N., Herrington, W. G., Haynes, R., Emberson, J., Baigent, C., & de Boer, I. H. (2020). Apolipoprotein B, Triglyceride-Rich Lipoproteins, and Risk of Cardiovascular Events in Persons with CKD. Clin J Am Soc Nephrol, 15(1), 47-60. https://doi.org/10.2215/cjn.07320619
- Lee, Y. H., Scharnitz, T. P., Muscat, J., Chen, A., Gupta-Elera, G., & Kirby, J. S. (2016). Laboratory Monitoring During Isotretinoin Therapy for Acne: A Systematic Review and Meta-analysis. JAMA Dermatol, 152(1), 35-44. https://doi.org/10.1001/jamadermatol.2015.3091
- Li, N., & Wang, J. A. (2005). Brain natriuretic peptide and optimal management of heart failure. J Zhejiang Univ Sci B, 6(9), 877-884. https://doi.org/10.1631/jzus.2005.B0877
- Liu, G., Dong, M., Ma, S., Fu, L., Xiao, Y., Zhong, L., & Geng, J. (2019). Serum leptin is associated with first-ever ischemic stroke, lesion size and stroke severity in a Chinese cohort. Neurol Res, 41(2), 125-131. https://doi.org/10.1080/01616412.2018.1544399
- LPSC. (2010). Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. The Lancet, 375(9725), 1536-1544. https://doi.org/10.1016/S0140-6736(10)60319-4
- Mach, F., Baigent, C., Catapano, A. L., Koskinas, K. C., Casula, M., Badimon, L., Chapman, M. J., De Backer, G. G., Delgado, V., Ference, B. A., Graham, I. M., Halliday, A., Landmesser, U., Mihaylova, B., Pedersen, T. R., Riccardi, G., Richter, D. J., Sabatine, M. S., Taskinen, M. R., Tokgozoglu, L., & Wiklund, O. (2019). 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. https://doi.org/10.1093/eurheartj/ehz455
- MacNamara, J., Eapen, D. J., Quyyumi, A., & Sperling, L. (2015). Novel biomarkers for cardiovascular risk assessment: current status and future directions. Future Cardiol, 11(5), 597-613. https://doi.org/10.2217/fca.15.39
- Maners, J., Gill, D., Pankratz, N., & Tang, W. (2019).
- Mark, D. B., Cowper, P. A., Anstrom, K. J., Sheng, S., Daniels, M. R., Knight, J. D., Baloch, K. N., Davidson-Ray, L., Fiuzat, M., Januzzi, J. L., Jr., Whellan, D. J., Piña, I. L., Ezekowitz, J. A., Adams, K. F., Cooper, L. S., O'Connor, C. M., & Felker, G. M. (2018). Economic and Quality-of-Life Outcomes of Natriuretic Peptide-Guided Therapy for Heart Failure. J Am Coll Cardiol, 72(21), 2551-2562. https://doi.org/10.1016/j.jacc.2018.08.2184
- Mehta, A., Virani, S. S., Ayers, C. R., Sun, W., Hoogeveen, R. C., Rohatgi, A., Berry, J. D., Joshi, P. H., Ballantyne, C. M., & Khera, A. (2020). Lipoprotein(a) and Family History Predict Cardiovascular Disease Risk. J Am Coll Cardiol, 76(7), 781-793. https://doi.org/10.1016/j.jacc.2020.06.040
- Mohler, E. R., 3rd, Ballantyne, C. M., Davidson, M. H., Hanefeld, M., Ruilope, L. M., Johnson, J. L., & Zalewski, A. (2008). The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J Am Coll Cardiol, 51(17), 1632-1641. https://doi.org/10.1016/j.jacc.2007.11.079
- Morita, S. Y. (2016). Metabolism and Modification of Apolipoprotein B-Containing Lipoproteins Involved in Dyslipidemia and Atherosclerosis. Biol Pharm Bull, 39(1), 1-24. https://doi.org/10.1248/bpb.b15-00716
- Moyer, V. A. (2013). Screening for primary hypertension in children and adolescents: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med, 159(9), 613-619. https://doi.org/10.7326/0003-4819-159-9-201311050-00725
- Mozaffarian, D. (2021, 12/17/2021). Fish oil: Physiologic effects and administration. Retrieved 5/11/2022 from https://www.uptodate.com/contents/fish-oil-and-marine-omega-3-fatty-acids?search=Omega-3%20Fatty%20Acid&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Musunuru, K., Ingelsson, E., Fornage, M., Liu, P., Murphy Anne, M., Newby, L. K., Newton-Cheh, C., Perez Marco, V., Voora, D., & Woo, D. (2017). The Expressed Genome in Cardiovascular Diseases and Stroke: Refinement, Diagnosis, and Prediction: A Scientific Statement From the American Heart Association. Circulation: Cardiovascular Genetics, 10(4), e000037. https://doi.org/10.1161/HCG.0000000000000037
- Newman, C. B., Blaha, M. J., Boord, J. B., Cariou, B., Chait, A., Fein, H. G., Ginsberg, H. N., Goldberg, I. J., Murad, M. H., Subramanian, S., & Tannock, L. R. (2020). Lipid Management in Patients with Endocrine Disorders: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism, 105(12), 3613-3682. https://doi.org/10.1210/clinem/dgaa674
- NICE. (2016). Cardiovascular disease: risk assessment and reduction, including lipid modification. https://www.nice.org.uk/guidance/cg181/chapter/1-Recommendations
- O'Malley, P. G., Arnold, M. J., Kelley, C., Spacek, L., Buelt, A., Natarajan, S., Donahue, M. P., Vagichev, E., Ballard-Hernandez, J., Logan, A., Thomas, L., Ritter, J., Neubauer, B. E., & Downs, J. R. (2020). Management of Dyslipidemia for Cardiovascular Disease Risk Reduction: Synopsis of the 2020 Updated U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann Intern Med, 173(10), 822-829. https://doi.org/10.7326/m20-4648
- Palomaki, G. E., Melillo, S., & Bradley, L. A. (2010). Association between 9p21 genomic markers and heart disease: a meta-analysis. Jama, 303(7), 648-656. https://doi.org/10.1001/jama.2010.118
- Palotie, A., Havulinna, A. S., De Livera, A. M., Tikkanen, E., Sijbrands, E. J., Abraham, G., Schunkert, H., Yetukuri, L., Perola, M., Bhalala, O. G., Byars, S. G., Inouye, M., Samani, N. J., Ripatti, S., & Salomaa, V. (2016). Genomic prediction of coronary heart disease. European Heart Journal, 37(43), 3267-3278. https://doi.org/10.1093/eurheartj/ehw450
- Patel, R. S., Asselbergs, F. W., Quyyumi, A. A., Palmer, T. M., Finan, C. I., Tragante, V., Deanfield, J., Hemingway, H., Hingorani, A. D., & Holmes, M. V. (2014). Genetic variants at chromosome 9p21 and risk of first versus subsequent coronary heart disease events: a systematic review and meta-analysis. J Am Coll Cardiol, 63(21), 2234-2245. https://doi.org/10.1016/j.jacc.2014.01.065
- Paynter, N. P., Chasman, D. I., Buring, J. E., Shiffman, D., Cook, N. R., & Ridker, P. M. (2009). Cardiovascular disease risk prediction with and without knowledge of genetic variation at chromosome 9p21.3. Ann Intern Med, 150(2), 65-72. http://dx.doi.org/
- Pearson, G. J., Thanassoulis, G., Anderson, T. J., Barry, A. R., Couture, P., Dayan, N., Francis, G. A., Genest, J., Grégoire, J., Grover, S. A., Gupta, M., Hegele, R. A., Lau, D., Leiter, L. A., Leung, A. A., Lonn, E., Mancini, G. B. J., Manjoo, P., McPherson, R., Ngui, D., Piché, M. E., Poirier, P., Sievenpiper, J., Stone, J., Ward, R., & Wray, W. (2021). 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can J Cardiol, 37(8), 1129-1150. https://doi.org/10.1016/j.cjca.2021.03.016
- Piepoli, M. F., Hoes, A. W., Agewall, S., Albus, C., Brotons, C., Catapano, A. L., Cooney, M. T., Corrà, U., Cosyns, B., Deaton, C., Graham, I., Hall, M. S., Hobbs, F. D. R., Løchen, M. L., Löllgen, H., Marques-Vidal, P., Perk, J., Prescott, E., Redon, J., Richter, D. J., Sattar, N., Smulders, Y., Tiberi, M., van der Worp, H. B., van Dis, I., Verschuren, W. M. M., & Binno, S. (2016). 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J, 37(29), 2315-2381. https://doi.org/10.1093/eurheartj/ehw106
- Pieters, M., Ferreira, M., de Maat, M. P. M., & Ricci, C. (2020). Biomarker association with cardiovascular disease and mortality - The role of fibrinogen. A report from the NHANES study. Thromb Res, 198, 182-189. https://doi.org/10.1016/j.thromres.2020.12.009
- Pignone, M. P. (2021, 11/4/2021). Management of elevated low density lipoprotein-cholesterol (LDL-C) in primary prevention of cardiovascular disease. Retrieved 5/11/2022 from https://www.uptodate.com/contents/management-of-elevated-low-density-lipoprotein-cholesterol-ldl-c-in-primary-prevention-of-cardiovascular-disease
- Pile, H. D., & Sadiq, N. M. (2019). Isotretinoin. In StatPearls. StatPearls Publishing
- StatPearls Publishing LLC. https://www.ncbi.nlm.nih.gov/pubmed/30247824
- Polcwiartek, C., Kragholm, K., Schjerning, O., Graff, C., & Nielsen, J. (2016). Cardiovascular safety of antipsychotics: a clinical overview. Expert Opin Drug Saf, 15(5), 679-688. https://doi.org/10.1517/14740338.2016.1161021
- Quest. (2019). Cardio IQ® 9p21 Genotype https://www.questdiagnostics.com/testcenter/TestDetail.action?ntc=90648&searchString=
- Reklou, A., Katsiki, N., Karagiannis, A., & Athyros, V. (2020). Effects of Lipid Lowering Drugs on Arterial Stiffness: One More Way to Reduce Cardiovascular Risk? Curr Vasc Pharmacol, 18(1), 38-42. https://doi.org/10.2174/1570161117666190121102323
- Ridker, P., MacFadyen, J., Glynn, R., & Chasman, D. (2011). Kinesin-Like Protein 6 (KIF6) Polymorphism and the Efficacy of Rosuvastatin in Primary Prevention. Circulation: Cardiovascular Genetics, 4(3), 312-317. https://doi.org/10.1161/CIRCGENETICS.110.959353
- Rizos, E. C., Ntzani, E. E., Bika, E., Kostapanos, M. S., & Elisaf, M. S. (2012). Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. Jama, 308(10), 1024-1033. https://doi.org/10.1001/2012.jama.11374
- Robinson, J. G., Williams, K. J., Gidding, S., Boren, J., Tabas, I., Fisher, E. A., Packard, C., Pencina, M., Fayad, Z. A., Mani, V., Rye, K. A., Nordestgaard, B. G., Tybjaerg-Hansen, A., Douglas, P. S., Nicholls, S. J., Pagidipati, N., & Sniderman, A. (2018). Eradicating the Burden of Atherosclerotic Cardiovascular Disease by Lowering Apolipoprotein B Lipoproteins Earlier in Life. J Am Heart Assoc, 7(20), e009778. https://doi.org/10.1161/jaha.118.009778
- Rosenberg, S., Elashoff, M. R., Beineke, P., Daniels, S. E., Wingrove, J. A., Tingley, W. G., Sager, P. T., Sehnert, A. J., Yau, M., Kraus, W. E., Newby, L. K., Schwartz, R. S., Voros, S., Ellis, S. G., Tahirkheli, N., Waksman, R., McPherson, J., Lansky, A., Winn, M. E., Schork, N. J., & Topol, E. J. (2010). Multicenter validation of the diagnostic accuracy of a blood-based gene expression test for assessing obstructive coronary artery disease in nondiabetic patients. Ann Intern Med, 153(7), 425-434. https://doi.org/10.7326/0003-4819-153-7-201010050-00005
- Rosenson, R. (2021, 8/19/2021). HDL cholesterol: Clinical aspects of abnormal values. Retrieved 5/11/2022 from https://www.uptodate.com/contents/hdl-cholesterol-clinical-aspects-of-abnormal-values?topicRef=4565&source=see_link
- Rosenson, R. (2022a, 1/18/2022). Lipoprotein classification, metabolism, and role in atherosclerosis. https://www.uptodate.com/contents/lipoprotein-classification-metabolism-and-role-in-atherosclerosis?search=apolipoprotein%20b&source=search_result&selectedTitle=1~67&usage_type=default&display_rank=1
- Rosenson, R. (2022b). Lipoprotein classification, metabolism, and role in atherosclerosis. Retrieved 5/11/2022 from https://www.uptodate.com/contents/lipoprotein-classification-metabolism-and-role-in-atherosclerosis?search=apolipoprotein%20b&source=search_result&selectedTitle=1~67&usage_type=default&display_rank=1
- Rosenson, R. (2022c, 1/31/2022). Lipoprotein(a). https://www.uptodate.com/contents/lipoprotein-a-and-cardiovascular-disease?source=see_link
- Rosenson, R. (2022d). Lipoprotein(a). Retrieved 5/11/2022 from https://www.uptodate.com/contents/lipoprotein-a-and-cardiovascular-disease?source=see_link
- Rosenson, R. (2022, 11/4/2021). Measurement of blood lipids and lipoproteins. Retrieved 5/11/2022 from https://www.uptodate.com/contents/measurement-of-blood-lipids-and-lipoproteins?search=lipid%20panel&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2
- Rosenson, R. S., Smith, C. Christopher, Bauer, Kenneth A. (2021, 12/6/2021). Overview of homocysteine. Retrieved 5/11/2022 from https://www.uptodate.com/contents/overview-of-homocysteine?search=Homocysteine&source=search_result&selectedTitle=1~149&usage_type=default&display_rank=1#H3163022070
- Rosenson, R. S., & Stafforini, D. M. (2012). Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J Lipid Res, 53(9), 1767-1782. https://doi.org/10.1194/jlr.R024190
- Rosenzweig, J. L., Bakris, G. L., Berglund, L. F., Hivert, M. F., Horton, E. S., Kalyani, R. R., Murad, M. H., & Verges, B. L. (2019). Primary Prevention of ASCVD and T2DM in Patients at Metabolic Risk: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2019-01338
- Rotella, F., Cassioli, E., Calderani, E., Lazzeretti, L., Ragghianti, B., Ricca, V., & Mannucci, E. (2020). Long-term metabolic and cardiovascular effects of antipsychotic drugs. A meta-analysis of randomized controlled trials. Eur Neuropsychopharmacol. https://doi.org/10.1016/j.euroneuro.2019.12.118
- Ruiz-Ramos, D., Hernández-Díaz, Y., Tovilla-Zárate, C. A., Juárez-Rojop, I., López-Narváez, M. L., González-Castro, T. B., Torres-Hernández, M. E., & Baños-González, M. A. (2015). The Trp719Arg polymorphism of the KIF6 gene and coronary heart disease risk: systematic review and meta-analysis. Hereditas, 152, 3. https://doi.org/10.1186/s41065-015-0004-7
- Rule, A., Glassock, Richard. (2020). The aging kidney. Retrieved 12/4/20 from https://www.uptodate.com/contents/the-aging-kidney?search=Cystatin%20C%20CVD&source=search_result&selectedTitle=4~150&usage_type=default&display_rank=4
- Samani, N. J., Erdmann, J., Hall, A. S., Hengstenberg, C., Mangino, M., Mayer, B., Dixon, R. J., Meitinger, T., Braund, P., Wichmann, H. E., Barrett, J. H., Konig, I. R., Stevens, S. E., Szymczak, S., Tregouet, D. A., Iles, M. M., Pahlke, F., Pollard, H., Lieb, W., Cambien, F., Fischer, M., Ouwehand, W., Blankenberg, S., Balmforth, A. J., Baessler, A., Ball, S. G., Strom, T. M., Braenne, I., Gieger, C., Deloukas, P., Tobin, M. D., Ziegler, A., Thompson, J. R., & Schunkert, H. (2007). Genomewide association analysis of coronary artery disease. N Engl J Med, 357(5), 443-453. https://doi.org/10.1056/NEJMoa072366
- Sandhu, P. K., Musaad, S. M., Remaley, A. T., Buehler, S. S., Strider, S., Derzon, J. H., Vesper, H. W., Ranne, A., Shaw, C. S., & Christenson, R. H. (2016). Lipoprotein Biomarkers and Risk of Cardiovascular Disease: A Laboratory Medicine Best Practices (LMBP) Systematic Review. J Appl Lab Med, 1(2), 214-229. https://doi.org/10.1373/jalm.2016.021006
- Sarnak, M., Gibson, Michael, Henrich, William. (2019). Chronic kidney disease and coronary heart disease. Retrieved 12/4/20 from https://www.uptodate.com/contents/chronic-kidney-disease-and-coronary-heart-disease
- SIGN. (2017). SIGN 149 • Risk estimation and the prevention of cardiovascular disease. https://www.sign.ac.uk/assets/sign149.pdf
- Singulex. (2019). Singulex Clinical Lab Blood Tests. http://dev1.singulex.boldfocus.com/blood-tests-descriptions/
- Siscovick, D. S., Barringer, T. A., Fretts, A. M., Wu, J. H., Lichtenstein, A. H., Costello, R. B., Kris-Etherton, P. M., Jacobson, T. A., Engler, M. B., Alger, H. M., Appel, L. J., & Mozaffarian, D. (2017). Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation, 135(15), e867-e884. https://doi.org/10.1161/cir.0000000000000482
- Sudhir, K. (2006). Lipoprotein-associated phospholipase A2, vascular inflammation and cardiovascular risk prediction. Vasc Health Risk Manag, 2(2), 153-156. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1994000/
- Superko, H. R., Superko, A. R., Lundberg, G. P., Margolis, B., Garrett, B. C., Nasir, K., & Agatston, A. S. (2014). Omega-3 Fatty Acid Blood Levels Clinical Significance Update. Curr Cardiovasc Risk Rep, 8(11). https://doi.org/10.1007/s12170-014-0407-4
- Suthahar, N., Meems, L. M. G., van Veldhuisen, D. J., Walter, J. E., Gansevoort, R. T., Heymans, S., Schroen, B., van der Harst, P., Kootstra-Ros, J. E., van Empel, V., Mueller, C., Bakker, S. J. L., & de Boer, R. A. (2020). High-Sensitivity Troponin-T and Cardiovascular Outcomes in the Community: Differences Between Women and Men. Mayo Clin Proc, 95(6), 1158-1168. https://doi.org/10.1016/j.mayocp.2020.01.017
- Tang, O., Matsushita, K., Coresh, J., Hoogeveen, R. C., Windham, B. G., Ballantyne, C. M., & Selvin, E. (2020). High-Sensitivity Cardiac Troponin I for Risk Stratification in Older Adults. J Am Geriatr Soc. https://doi.org/10.1111/jgs.16912
- Tedeschi-Reiner, E., Strozzi, M., Skoric, B., & Reiner, Z. (2005). Relation of atherosclerotic changes in retinal arteries to the extent of coronary artery disease. Am J Cardiol, 96(8), 1107-1109. https://doi.org/10.1016/j.amjcard.2005.05.070
- Thomas, G., Voros, S., McPherson, J., Lansky, A., Winn, M. E., Bateman, T., Elashoff, M. R., Lieu, H., Johnson, A., Daniels, S. E., Ladapo, J., Phelps, C., Douglas, P., & Rosenberg, S. (2013). A Blood-Based Gene Expression Test for Obstructive Coronary Artery Disease Tested in Symptomatic Nondiabetic Patients Referred for Myocardial Perfusion Imaging The COMPASS Study. Circulation: Cardiovascular Genetics, 6(2), 154-162. https://doi.org/10.1161/CIRCGENETICS.112.964015
- Thompson, M. A., Horberg, M. A., Agwu, A. L., Colasanti, J. A., Jain, M. K., Short, W. R., Singh, T., & Aberg, J. A. (2020). Primary Care Guidance for Persons With Human Immunodeficiency Virus: 2020 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical Infectious Diseases, 73(11), e3572-e3605. https://doi.org/10.1093/cid/ciaa1391
- Tobe, S. W., Stone, J. A., Anderson, T., Bacon, S., Cheng, A. Y. Y., Daskalopoulou, S. S., Ezekowitz, J. A., Gregoire, J. C., Gubitz, G., Jain, R., Keshavjee, K., Lindsay, P., L'Abbe, M., Lau, D. C. W., Leiter, L. A., O'Meara, E., Pearson, G. J., Rabi, D. M., Sherifali, D., Selby, P., Tu, J. V., Wharton, S., Walker, K. M., Hua-Stewart, D., & Liu, P. P. (2018). Canadian Cardiovascular Harmonized National Guidelines Endeavour (C-CHANGE) guideline for the prevention and management of cardiovascular disease in primary care: 2018 update. Cmaj, 190(40), E1192-e1206. https://doi.org/10.1503/cmaj.180194
- Tomcsányi, J., Somlói, M., Bózsik, B., Frész, T., & Nagy, E. (2018). [The value of early repeated N-terminal pro-B-type natriuretic peptide measurement in acute heart failure]. Orv Hetil, 159(25), 1009-1012. https://doi.org/10.1556/650.2018.31095 (Az N-terminális pro-B natriureticus peptid mérésének korai ismétlése akut szívelégtelenség miatt hospitalizált betegeken.)
- Trenkwalder, T., Nelson, C. P., Musameh, M. D., Mordi, I. R., Kessler, T., Pellegrini, C., Debiec, R., Rheude, T., Lazovic, V., Zeng, L., Martinsson, A., Gustav Smith, J., Gadin, J. R., Franco-Cereceda, A., Eriksson, P., Nielsen, J. B., Graham, S. E., Willer, C. J., Hveem, K., Kastrati, A., Braund, P. S., Palmer, C. N. A., Aracil, A., Husser, O., Koenig, W., Schunkert, H., Lang, C. C., Hengstenberg, C., & Samani, N. J. (2019). Effects of the coronary artery disease associated LPA and 9p21 loci on risk of aortic valve stenosis. Int J Cardiol, 276, 212-217. https://doi.org/10.1016/j.ijcard.2018.11.094
- Trompet, S., Packard, C. J., & Jukema, J. W. (2018). Plasma apolipoprotein-B is an important risk factor for cardiovascular disease, and its assessment should be routine clinical practice. Curr Opin Lipidol, 29(1), 51-52. https://doi.org/10.1097/mol.0000000000000476
- USPSTF. (2015). Screening for abnormal blood glucose and type 2 diabetes mellitus: U.s. preventive services task force recommendation statement. Ann Intern Med, 163(11), 861-868. https://doi.org/10.7326/M15-2345
- USPSTF. (2018a). Risk assessment for cardiovascular disease with nontraditional risk factors: Us preventive services task force recommendation statement. Jama, 320(3), 272-280. https://doi.org/10.1001/jama.2018.8359
- USPSTF. (2018b). Screening for Cardiovascular Disease Risk With Electrocardiography: US Preventive Services Task Force Recommendation Statement. Jama, 319(22), 2308-2314. https://doi.org/10.1001/jama.2018.6848
- USPSTF. (2020). High Blood Pressure in Children and Adolescents: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
- USPSTF. (2021). Hypertension in Adults: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
- Varbo, A., Benn, M., & Nordestgaard, B. G. (2014). Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther, 141(3), 358-367. https://doi.org/10.1016/j.pharmthera.2013.11.008
- Varbo, A., Benn, M., Tybjaerg-Hansen, A., Jorgensen, A. B., Frikke-Schmidt, R., & Nordestgaard, B. G. (2013). Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol, 61(4), 427-436. https://doi.org/10.1016/j.jacc.2012.08.1026
- Virani Salim, S., Alonso, A., Benjamin Emelia, J., Bittencourt Marcio, S., Callaway Clifton, W., Carson April, P., Chamberlain Alanna, M., Chang Alexander, R., Cheng, S., Delling Francesca, N., Djousse, L., Elkind Mitchell, S. V., Ferguson Jane, F., Fornage, M., Khan Sadiya, S., Kissela Brett, M., Knutson Kristen, L., Kwan Tak, W., Lackland Daniel, T., Lewis Tené, T., Lichtman Judith, H., Longenecker Chris, T., Loop Matthew, S., Lutsey Pamela, L., Martin Seth, S., Matsushita, K., Moran Andrew, E., Mussolino Michael, E., Perak Amanda, M., Rosamond Wayne, D., Roth Gregory, A., Sampson Uchechukwu, K. A., Satou Gary, M., Schroeder Emily, B., Shah Svati, H., Shay Christina, M., Spartano Nicole, L., Stokes, A., Tirschwell David, L., VanWagner Lisa, B., Tsao Connie, W., & null, n. (2020). Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation, 141(9), e139-e596. https://doi.org/10.1161/CIR.0000000000000757
- Virani, S. S., Brautbar, A., Lee, V. V., Elayda, M., Morrison, A. C., Grove, M. L., Nambi, V., Frazier, L., Wilson, J. M., Willerson, J. T., Boerwinkle, E., & Ballantyne, C. M. (2012). Chromosome 9p21 Single Nucleotide Polymorphisms are Not Associated with Recurrent Myocardial Infarction in Patients with Established Coronary Artery Disease. Circ J, 76(4), 950-956. http://dx.doi.org/
- Visseren, F. L. J., Mach, F., Smulders, Y. M., Carballo, D., Koskinas, K. C., Bäck, M., Benetos, A., Biffi, A., Boavida, J. M., Capodanno, D., Cosyns, B., Crawford, C., Davos, C. H., Desormais, I., Di Angelantonio, E., Franco, O. H., Halvorsen, S., Hobbs, F. D. R., Hollander, M., Jankowska, E. A., Michal, M., Sacco, S., Sattar, N., Tokgozoglu, L., Tonstad, S., Tsioufis, K. P., van Dis, I., van Gelder, I. C., Wanner, C., & Williams, B. (2021). 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J, 42(34), 3227-3337. https://doi.org/10.1093/eurheartj/ehab484
- Visseren, F. L. J., Mach, F., Smulders, Y. M., Carballo, D., Koskinas, K. C., Back, M., Benetos, A., Biffi, A., Boavida, J. M., Capodanno, D., Cosyns, B., Crawford, C., Davos, C. H., Desormais, I., Di Angelantonio, E., Franco, O. H., Halvorsen, S., Hobbs, F. D. R., Hollander, M., Jankowska, E. A., Michal, M., Sacco, S., Sattar, N., Tokgozoglu, L., Tonstad, S., Tsioufis, K. P., van Dis, I., van Gelder, I. C., Wanner, C., Williams, B., Societies, E. S. C. N. C., & Group, E. S. C. S. D. (2021). 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J, 42(34), 3227-3337. https://doi.org/10.1093/eurheartj/ehab484
- WellnessFX. (2021). Your Wellness In Your Hands. Retrieved 1/4/2021 from https://www.wellnessfx.com/
- Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. E., Jr., Collins, K. J., Dennison Himmelfarb, C., DePalma, S. M., Gidding, S., Jamerson, K. A., Jones, D. W., MacLaughlin, E. J., Muntner, P., Ovbiagele, B., Smith, S. C., Jr., Spencer, C. C., Stafford, R. S., Taler, S. J., Thomas, R. J., Williams, K. A., Sr., Williamson, J. D., & Wright, J. T., Jr. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension, 71(6), 1269-1324. https://doi.org/10.1161/hyp.0000000000000066
- Willeit, P., Ridker, P. M., Nestel, P. J., Simes, J., Tonkin, A. M., Pedersen, T. R., Schwartz, G. G., Olsson, A. G., Colhoun, H. M., Kronenberg, F., Drechsler, C., Wanner, C., Mora, S., Lesogor, A., & Tsimikas, S. (2018). Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet, 392(10155), 1311-1320. https://doi.org/10.1016/s0140-6736(18)31652-0
- Wilson, D. P., Jacobson, T. A., Jones, P. H., Koschinsky, M. L., McNeal, C. J., Nordestgaard, B. G., & Orringer, C. E. (2019). Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol, 13(3), 374-392. https://doi.org/10.1016/j.jacl.2019.04.010
- Wilson, P. (2017). Overview of the possible risk factors for cardiovascular disease - UpToDate. In B. Downey (Ed.), UpToDate. https://www.uptodate.com/contents/overview-of-the-possible-risk-factors-for-cardiovascular-disease?source=search_result&search=genetic%20cardiovascular%20risk&selectedTitle=1~150#H1267867
- Wilson, P. (2020a). Cardiovascular disease risk assessment for primary prevention: Our approach. Retrieved 12/4/20 from https://www.uptodate.com/contents/cardiovascular-disease-risk-assessment-for-primary-prevention-our-approach?search=biomarkers%20CVD&source=search_result&selectedTitle=5~150&usage_type=default&display_rank=5
- Wilson, P. (2020b). Overview of the possible risk factors for cardiovascular disease - UpToDate. In B. Downey (Ed.), UpToDate. https://www.uptodate.com/contents/overview-of-the-possible-risk-factors-for-cardiovascular-disease?source=search_result&search=genetic%20cardiovascular%20risk&selectedTitle=1~150#H1267867
- Wong, N. D., Budoff, M. J., Ferdinand, K., Graham, I. M., Michos, E. D., Reddy, T., Shapiro, M. D., & Toth, P. P. (2022). Atherosclerotic cardiovascular disease risk assessment: An American Society for Preventive Cardiology clinical practice statement. Am J Prev Cardiol, 10, 100335. https://doi.org/10.1016/j.ajpc.2022.100335
- Yang, H., Guo, W., Li, J., Cao, S., Zhang, J., Pan, J., Wang, Z., Wen, P., Shi, X., & Zhang, S. (2017). Leptin concentration and risk of coronary heart disease and stroke: A systematic review and meta-analysis. PLoS One, 12(3), e0166360. https://doi.org/10.1371/journal.pone.0166360
- Zane, L. T., Leyden, W. A., Marqueling, A. L., & Manos, M. M. (2006). A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol, 142(8), 1016-1022. https://doi.org/10.1001/archderm.142.8.1016
- Zhernakova, D. V., Le, T. H., Kurilshikov, A., Atanasovska, B., Bonder, M. J., Sanna, S., Claringbould, A., Võsa, U., Deelen, P., Franke, L., de Boer, R. A., Kuipers, F., Netea, M. G., Hofker, M. H., Wijmenga, C., Zhernakova, A., Fu, J., LifeLines cohort, s., & consortium, B. (2018). Individual variations in cardiovascular-disease-related protein levels are driven by genetics and gut microbiome. Nature Genetics, 50(11), 1524-1532. https://doi.org/10.1038/s41588-018-0224-7
Coding Section
|
CPT |
Code Description |
|
80061 |
Lipid panel This panel must include the following: Cholesterol, serum, total (82465) Lipoprotein, direct measurement, high density cholesterol (HDL cholesterol) (83718) Triglycerides (84478) |
|
81402 |
Molecular pathology procedure, Level 3 (e.g., > 10 SNPs, 2-10 methylated variants, or 2 – 10 somatic variants [typically using non-sequencing target variant analysis], immunoglobulin and T-cell receptor gene rearrangements, duplication/deletion variants of 1 exon, loss of heterozygosity [LOH], uniparental disomy [UPD]) |
|
81403 |
Molecular pathology procedure, Level 4 (e.g., analysis of single exon by DNA sequence analysis, analysis of > 10 amplicons using multiplex PCR in 2 or more independent reactions, mutation scanning or duplication/deletion variants of 2 – 5 exons) |
|
81404 |
Molecular pathology procedure, Level 5 (e.g., analysis of 2 – 5 exons by DNA sequence analysis, mutation scanning or duplication/deletion variants of 6-10 exons, or characterization of a dynamic mutation disorder/triplet repeat by Southern blot analysis) |
|
81405 |
Molecular pathology procedure, Level 6 (e.g., analysis of 6 – 10 exons by DNA sequence analysis, mutation scanning or duplication/deletion variants of 11 – 25 exons, regionally targeted cytogenomic array analysis) |
|
81406 |
Molecular pathology procedure, Level 7 (e.g., analysis of 11-25 exons by DNA sequence analysis, mutation scanning or duplication/deletion variants of 26-50 exons, cytogenomic array analysis for neoplasia) |
|
81407 |
Molecular pathology procedure, Level 8 (e.g., analysis of 26-50 exons by DNA sequence analysis, mutation scanning or duplication/deletion variants of >50 exons, sequence analysis of multiple genes on one platform) |
|
81408 |
Molecular pathology procedure, Level 9 (e.g., analysis of > 50 exons in a single gene by DNA sequence analysis) |
|
81479 |
Unlisted molecular pathology procedure |
|
81493 |
Coronary artery disease, mRNA, gene expression profiling by real-time RT-PCR of 23 genes, utilizing whole peripheral blood, algorithm reported as a risk score |
|
81599 |
Unlisted multianalyte assay with algorithmic analysis |
|
82172 |
Apolipoprotein, each |
|
82465 |
Cholesterol, serum or whole blood, total |
|
82610 |
Cystatin C |
|
83090 |
Homocysteine |
|
83695 |
Lipoprotein (a) |
|
83698 |
Lipoprotein-associated phospholipase A2 (Lp-PLA2) |
|
83700 |
Lipoprotein, blood; electrophoretic separation and quantitation |
|
83701 |
Lipoprotein, blood; high resolution fractionation and quantitation of lipoproteins including lipoprotein subclasses when performed (e.g., electrophoresis, ultracentrifugation) |
|
83704 |
Lipoprotein, blood; quantitation of lipoprotein particle number(s) (e.g., by nuclear magnetic resonance spectroscopy), includes lipoprotein particle subclass(es), when performed |
|
83718 |
Lipoprotein, direct measurement; high density cholesterol (HDL cholesterol) |
|
83719 |
Lipoprotein, direct measurement; VLDL cholesterol |
|
83721 |
Lipoprotein, direct measurement; LDL cholesterol |
|
83722 |
Lipoprotein, direct measurement; small dense LDL cholesterol |
|
83880 |
Natriuretic peptide |
|
84478 |
Triglycerides |
|
84484 |
Troponin, quantitative |
|
84512 |
Troponin, qualitative |
|
84999 |
Unlisted chemistry procedure |
|
85384 |
Fibrinogen; activity |
|
85415 |
Fibrinolytic factors and inhibitors; plasminogen activator |
|
86141 |
C-reactive protein; high sensitivity (hsCRP) |
|
0052U |
Lipoprotein, blood, high resolution fractionation and quantitation of lipoproteins, including all five major lipoprotein classes and subclasses of HDL, LDL, and VLDL by vertical auto profile ultracentrifugation |
|
0308U |
Cardiology (coronary artery disease [CAD]), analysis of 3 proteins (high sensitivity [hs] troponin, adiponectin, and kidney injury molecule-1 [KIM-1]), plasma, algorithm reported as a risk score for obstructive CAD |
|
0309U |
Cardiology (cardiovascular disease), analysis of 4 proteins (NT-proBNP, osteopontin, tissue inhibitor of metalloproteinase-1 [TIMP-1], and kidney injury molecule-1 [KIM-1]), plasma, algorithm reported as a risk score for major adverse cardiac event |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, Blue Cross Blue Shield Association technology assessment program (TEC) and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2018 Forward
| 04/05/2023 | Change review date to coincide with Avalon review date. No changes made to policy.. |
| 08/10/2022 |
Entire policy is being updated as all genetic testing is being migrated to a new policy. Only non genetic testing will remain in the policy, but, there is no change to the intent of that testing. Updating description, policy, rationale, references and coding. |
|
04/27/2022 |
Annual review, multiple coverage criteria, , note 1, rationale, references and coding. |
|
07/08/2021 |
Interim review updating coding. No other changes. |
|
04/07/2021 |
Annual review, changing age range for lipid panels from 20-79 years to 18-79 years. Also adding multiple conditions in the medical necessity criteria statement. Updating description, rationale, references and coding. |
|
10/01/2020 |
Interim review, removed "and management" from medical criteria number 8 novel cardiovascular biomarkers. No other changes made. |
|
07/01/2020 |
Interim review, major revision for clarity, adding medical necessity criteria for lipoprotein a testing. |
|
04/21/2020 |
Annual review, updating policy to include medical criteria for lipid panels for individuals on certain long term medications and apolipoprotein V for members with specific diagnoses. Also updating coding. |
|
04/03/2019 |
Annual review, adding medical necessity criteria for high sensitivity C reactive protein (hs-CRP) testing. Also updating coding. |
|
12/19/2018 |
Updating with 2019 codes. |
|
04/18/2018 |
Interim review adding investigational statements relating to 9p21 and KIF6 testing. No other changes made. |
|
02/28/2018 |
Updating coding with E72.11 and Z51.89. No other changes made. |
|
01/24/2018 |
New Policy |