Cervical Cancer Screening Technologies With Pap and HPV - CAM 314
Description
Cervical cancer screening detects cervical precancerous lesions and cancer through cytology, human papillomavirus (HPV) testing, and if needed, colposcopy (Feldman, Goodman, & Peipert, 2021). The principal screening test to detect cancer in asymptomatic women is the Papanicolaou (Pap) smear. It involves cells being scraped from the cervix during a pelvic examination and spread onto a slide. The slide is then sent to an accredited laboratory to be stained, observed, and interpreted (Feldman & Crum, 2021).
Human papilloma virus (HPV) has been associated with development of cervical intraepithelial neoplasia, and FDA approved HPV tests detecting the presence of viral DNA from high risk strains have been developed and validated as an adjunct primary cancer screening method (Feldman & Crum, 2019).
For more information specifically regarding HPV, please refer to AHS-G2157 Diagnostic Testing of STIs.
Regulatory Status
Several liquid based preparations have received premarket approval from the U.S. Food and Drug Administration (FDA). For example, in May 1996, "ThinPrep® Pap Test" was approved by the FDA through the premarket approval process for use n collecting and preparing cervical cytology specimens for Pap stain-based screening for cervical cancer.
Several automated screening systems have received premarket approval through FDA. For example, in September 1995, "AutoPap® Automatic Pap Screener, now FocalPointTM was approved by the FDA through the premarket approval process for use in initial screening of cervical cytology slides. The device is intended to be used on both conventionally prepared and prepstain system cervical cytology studies.
In March 2003, test kit "digene® HPV test" was approved by the FDA through premarket approval process for use in diagnostic testing for the qualitative detection of DNA from 13 high-risk papillomavirus types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) in cervical specimens.
In March 2009, test kit "Cervista® HPV HR was approved by the FDA through the premarket approval process for use in diagnostic testing for the qualitative detection of DNA from 14 high-risk papillomavirus (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) in cervical specimens.
Several organizations and professional societies have developed guidelines or recommendations for cervical cancer screening, to optimize early detection and to reduce false positives. The focus has been on test selection and frequency of testing, as well as follow-up testing for abnormal results.
In 2012, the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) issued joint guidelines regarding cervical cancer screening.1 The focus areas of their recommendations were listed as:
- Optimal cytology screening intervals
- Screening strategies for women 30 years and older
- Management of discordant combinations of cytology and HPV results (e.g., HPV positive, cytology negative and HPV negative, atypical squamous cells of undetermined significance [ASCUS] results)
- Exiting women from screening
- Impact of HPV vaccination on future screening practices
- Potential utility of molecular screening (Specifically, HPV testing for primary screening was assessed as a potential future strategy.)"
The recommendations are segregated primarily by age group:
- Women under 21 years of age (regardless of sexual activity or other high risk behavior), or over 65 years of age, or who have had surgical removal of the cervix, should not receive cervical cancer screening tests, unless there is a compelling medical reason to do so.
- Women 21 – 29 years of age should have screening with cervical cytology only, at a frequency of every 3 years. Cytology results of ASCUS in this group should be followed up by high risk HPV testing for determination of the appropriate follow-up.
- Women 30 – 65 years of age should preferably have co-testing with cervical cytology plus high-risk HPV testing every five years, or testing with cervical cytology alone every 3 years (deemed acceptable).
- Individuals who have been vaccinated against high risk HPV types should follow the testing recommendations for their age group.
- HPV testing alone (without cytology) is not recommended as a first tier cervical cancer screening test in any age group.
Also in 2014, the U.S. Preventive Services Task Force issued an update to its 2012 recommendation statement on cervical cancer screening.2 The criteria in the USPSTF recommendations are consistent with those listed above for the ACS, ASCCP, ASCP recommendation, except that the USPSTF does not indicate a preference for co-testing with Pap and HPV every 5 years over testing with Pap alone every 3 years, for women ages 30 – 65.
The USPSTF report notes that women have historically used the annual Pap test visit to discuss other health concerns with their health care provider. They urge that, "Individuals, clinicians, and health systems should seek effective ways to facilitate the receipt of recommended preventive services at intervals that are beneficial to the patient. Efforts should also be made to ensure that individuals are able to seek care for additional health concerns as they present."
Similarly, the American College of Obstetricians and Gynecologists issued a Practice Bulletin in 2012, which aligns with the recommendations above.4 Members of ACOG participated in the development of both sets of guidelines.
Referral for cancer genetic consultation is recommended by the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors for individuals with a personal or family history indicative of a hereditary form of cancer.
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
The criteria below are based on recommendations by the U.S. Preventive Services Task Force, The National Cancer Institute, NCCN, The American Society for Colposcopy and Cervical Pathology, The American Cancer Society, The American Society for Clinical Pathology, and the American College of Obstetricians and Gynecologists. Within these coverage criteria, “individual(s)” is specific to individuals with a cervix.
- In individuals who are under 21 years of age, cervical cancer screening is considered MEDICALLY NECESSARY only when one of the following criteria are met:
- There is a history of HIV and/or other non-HIV immunocompromised conditions.
- There is a previous diagnosis of cervical cancer.
- There is a previous diagnosis of cervical dysplasia.
- There is a history of an organ transplant.
- In immunosuppressed individuals without an HIV infection, any one of the following cervical cancer screening techniques is considered MEDICALLY NECESSARY:
- Annual cytology testing for individuals 30 years or younger.
- Every 3 years co-testing (cytology and HPV) for individuals 30 years or older.
- For individuals 21 – 29 years of age, cervical cancer screening using conventional or liquid-based Papanicolaou (Pap) smears is considered MEDICALLY NECESSARY at a frequency of every 3 years.
- For individuals 30 – 65 years of age, any one of the following cervical cancer screening techniques is considered MEDICALLY NECESSARY:
- Conventional or liquid-based Pap smear at a frequency of every 3 years.
- Cervical cancer screening using the high-risk HPV test alone at a frequency of every 5 years.
- Co-testing (cytology with concurrent high-risk HPV testing) at a frequency of every 5 years.
- For individuals who are HPV positive and cytology negative, testing for high-risk strains HPV-16 and HPV-18 is considered MEDICALLY NECESSARY.
- For individuals >65 years of age who are considered high-risk (women with a high-grade precancerous lesion or cervical cancer, women with in utero exposure to diethylstilbestrol, or women who are immunocompromised), cervical cancer screening is considered MEDICALLY NECESSARY.
- Repeat cervical cancer screening by Pap smear or HPV testing in one year is considered MEDICALLY NECESSARY in the following individuals:
- For individuals who had a previous cervical cancer screen with an abnormal cytology result and/or who was positive for HPV.
- For individuals at high risk for cervical cancer (organ transplant, exposure to the drug DES, immunocompromised individuals).
- For individuals > 65 years of age who are not considered high-risk and who have an adequate screening history, routine cervical cancer screening is considered NOT MEDICALLY NECESSARY. Adequate screening history is defined as either:
- Having three consecutive negative Pap smears.
- Having two consecutive negative HPV tests within 10 years before cessation of screening, with the most recent test occurring within 5 years.
- For individuals who have undergone surgical removal of the uterus and cervix and who have no history of cervical cancer or pre-cancer, cervical cancer screening (at any age) is considered NOT MEDICALLY NECESSARY.
The following does not meet coverage criteria due to a lack of available published scientific literature confirming that the test(s) is/are required and beneficial for the diagnosis and treatment of a patient’s illness.
- The following is considered NOT MEDICALLY NECESSARY:
- Inclusion of low-risk strains of HPV in co-testing, as the clinical utility has not been established.
- Other technologies for cervical cancer screening because of insufficient evidence of clinical utility.
Policy Guidelines
CPT codes 88142 – 88143 and 88174 – 88175 refer to the use of monolayer preparation (i.e., collected in preservative fluid) with various different screening options. CPT codes 88147 and 88148 refer to conventionally prepared slides with various different screening options while 88152, 88166, and 88167 refer to conventionally prepared slides with computer assisted rescreening with or without cell selection.
CPT codes 87620 – 87622 refer to the DNA probe technique for detecting HPV.
Benefit Application
BlueCard/National Account Issues
State or federal mandates (e.g., FEP) may dictate that all FDA-approved devices, drugs, or biologics may not be considered investigational and, thus may only be assessed on the basis of their medical necessity.
Rationale
The American Cancer Society estimates that 14,480 new cases of cervical cancer will be diagnosed in 2021 and approximately 4,290 women will die from the disease (ACS, 2021). To screen for cervical cancer, a Papanicolaou (Pap) test or human papillomavirus (HPV) test is performed. Co-testing with both is also a common clinical practice. To obtain the cell sample for cytology, cells are scraped from both the ectocervix (external surface) and endocervix (cervical canal) during a speculum exam to evaluate the squamocolumnar junction where most neoplasia occur. Cytological examination can be performed as either a traditional Pap smear where the swab is rolled directly on the slide for observation or as a liquid-based thin layer cytology examination where the swab is swirled in a liquid solution so that the free cells can be trapped and plated as a monolayer on the glass slide. One advantage of the liquid cytology assay is that the same sample can be used for HPV testing whereas a traditional Pap smear requires a second sample to be taken. HPV testing is typically a nucleic acid-based assay that checks for the presence of high-risk types of HPV, especially types 16 and 18. HPV testing can be performed on samples obtained during a cervical exam; furthermore, testing can be performed on samples obtained from a tampon, Dacron or cotton swab, cytobrush, or cervicovaginal lavage (Feldman & Crum, 2022).
Analytical Validity
A study by Marchand et al. (2005) explored the optimal collection technique for Pap testing. Their study consisted of two different cytology labs and 128 clinicians over the course of one year. They discovered that in conventional Pap testing the sequence of collection — the cytobrush for the endocervix and the spatula for the ectocervix — had no effect on the quality of the assay. Further, 47% of the clinicians who had high levels of absent endocervical cells on their samples used the cytobrush method alone. The authors conclude, “The combination of the Cytobrush (endocervix) and spatula (ectocervix) is superior for a quality Pap smear. The sequence of collection was not important in conventional Pap smears. The broom alone performs poorly (Marchand et al., 2005).”
Urine-based HPV DNA testing as a screening tool would be a less invasive method than cervical examinations and swabs. A study by Mendez et al. (2014) using both urine samples and cervical swabs from 52 female patients, however, showed that there was only 76% agreement between the two methodologies. The urine testing correctly identified 100% of the uninfected individuals but only 65% of the infected as compared to the cervical swab controls (Mendez et al., 2014). An extensive meta-analysis of 14 different studies using urinary testing, on the other hand, reported an 87% sensitivity and 94% specificity of the urine-based methodology for all strains of HPV, but the sensitivity for high-risk strains alone was only 77%. The specificity for the high-risk strains alone was reported to be higher at 98%. “The major limitations of this review are the lack of a strictly uniform method for the detection of HPV in urine and the variation in accuracy between individual studies. Testing urine for HPV seems to have good accuracy for the detection of cervical HPV and testing first void urine samples is more accurate than random or midstream sampling. When cervical HPV detection is considered difficult in certain subgroups, urine testing should be regarded as an acceptable alternative” (Pathak et al., 2014).
Clinical Utility and Validity
The National Cancer Institute (NCI) reports that “Regular Pap screening decreases cervix cancer incidence and mortality by at least 80%” (NCI, 2021). They also note that Pap testing can result in the possibility of additional diagnostic testing, especially in younger women, when unwarranted, especially in cases of possible low-grade squamous intraepithelial lesions (LSILs); however, even though 50% of women undergoing Pap testing required additional, follow-up diagnostic procedures, only 5% were treated for LSILs. The NCI also reports that “HPV-based screening provides 60% to 70% greater protection against invasive cervical carcinoma, compared with cytology” (NCI, 2021).
A study by Sabeena et al. (2019) measured the utility of urine-based sampling for cervical cancer screening in low-resource settings. The researchers compared 114 samples to determine the accuracy of HPV detection (by polymerase chain reaction (PCR)) in paired cervical and urine samples. Samples were taken from patients previously diagnosed with cervical cancer through histological methods. Of the 114 samples, “HPV DNA was tested positive in cervical samples of 89 (78.1%) and urine samples of 55 (48.2%) patients. The agreement between the two sampling methods was 66.7%” (Sabeena et al., 2019). HPV detection in urine samples had a sensitivity of 59.6% and a specificity of 92%. The authors concluded, “Even though not acceptable as an HPV DNA screening tool due to low sensitivity, the urine sampling method is inexpensive and more socially acceptable for large epidemiological surveys in developing countries to estimate the burden” (Sabeena et al., 2019).
Cervical cancer guidelines published by the National Comprehensive Cancer Network (NCCN) (NCCN, 2021) state that, although the rates of both incidence and mortality of squamous cell carcinoma of the cervix has been declining over the last thirty years, “adenocarcinoma of the cervix has increased over the past 3 decades, probably because cervical cytologic screening methods are less effective for adenocarcinoma.” A study in the United Kingdom supports this because the risk-reduction associated with 3-yearly screening was reduced by 75% for squamous carcinoma and 83% for adenosquamous carcinoma, but adenocarcinoma was reduced only by 43% (Sasieni et al., 2009). Another extensive study of more than 900,000 women in Sweden showed that PCR-based HPV testing for the high-risk types 16 and 18 is better at predicting the risk of both in situ and invasive adenocarcinoma. The authors conclude, “infections with HPV 16 and 18 are detectable up to at least 14 years before diagnosis of cervical adenocarcinoma. Our data provide prospective evidence that the association of HPV 16/18 with cervical adenocarcinoma is strong and causal (Dahlstrom et al., 2010).”
A report by Chen et al. (2011) reviewed HPV testing and the risk of the development of cervical cancer. Of the 11,923 women participating in the study, 86% of the women who tested positive for HPV did not develop cervical cancer with ten years. The authors concluded, “HPV negativity was associated with a very low long-term risk of cervical cancer. Persistent detection of HPV among cytologically normal women greatly increased risk. Thus, it is useful to perform repeated HPV testing following an initial positive test” (Chen et al., 2011).
In 2018, the results of the multi-year HPV for cervical cancer screening trial (FOCAL) randomized clinical trial testing of the use of HPV testing alone for detection of cervical intraepithelial neoplasia (CIN) grade 3 or worse (CIN3+) were published. More than 19,000 women participated in the study split between the intervention group (HPV testing alone) and the control group (liquid-based cytology). “Baseline HPV-negative women had a significantly lower cumulative incidence of CIN3+ at 48 months than cytology-negative women (CIN3+ incidence rate, 1.4/1000 [95% CI, 0.8 – 2.4]; CIN3+ risk ratio, 0.25 [95% CI, 0.13 – 0.48]). Among women undergoing cervical cancer screening, the use of primary HPV testing compared with cytology testing resulted in a significantly lower likelihood of CIN3+ at 48 months. Further research is needed to understand long-term clinical outcomes as well as cost-effectiveness (Ogilvie et al., 2018).” In a commentary concerning the findings of this trial, the author notes that “multiple randomized trials have shown that primary HPV screening linked to subsequent identification and treatment of cervical precancer is more effective than Pap testing in reducing the incidence of cervical cancer and precancer, at the cost of lower specificity and more false-negative subsequent colposcopic assessments (Massad, 2018).” The author does address the limitations of the FOCAL study, including that the study concluded prior to seeing what effects, if any, women vaccinated against HPV 16 and HPV 18 would have since the adolescents vaccinated upon FDA approval of the vaccine would not have necessarily been included within the study. They also state that a limitation of the FOCAL trial is “the use of a pooled HPV test for screening, incorporating all carcinogenic HPV types in a single positive or negative result” (Massad, 2018).
Melnikow et al. (2018) performed a review for the USPSTF regarding cervical cancer screening through high-risk (hr) HPV testing. The authors reviewed the following studies: “8 randomized clinical trials (n = 410,556), 5 cohort studies (n = 402,615), and 1 individual participant data (IPD) meta-analysis (n = 176,464).” Primary hr-HPV testing was found to detect cervical intraepithelial neoplasia (CIN) 3+ at an increased rate (relative risk rate ranging from 1.61 to 7.46) in round 1 screening. False positive rates for primary hr-HPV testing ranged from 6.6% to 7.4%, compared with 2.6% to 6.5% for cytology, whereas in cotesting, false-positives ranged from 5.8% to 19.9% in the first round of screening, compared with 2.6% to 10.9% for cytology. Overall, the authors concluded that “primary hrHPV screening detected higher rates of CIN 3+ at first-round screening compared with cytology. Cotesting trials did not show initial increased CIN 3+ detection” (Melnikow et al., 2018).
Bonde et al. (2020) performed a systematic review on the clinical utility of HPV genotyping as a form of cervical cancer screening. Through 16 studies, the researchers concluded that “HPV genotyping can refine clinical management for women screened through the primary HPV paradigm and the co-testing paradigm by stratifying genotype-specific results and thereby assign women at highest risk for cervical disease to further testing (i.e., colposcopy) or treatment, while designating those with lowest risk to retesting at a shortened interval.” After deeming low risk of bias, the review also stated “the overall quality of evidence for CIN 3 or worse risk with negative for intraepithelial lesions or malignancies or low-grade squamous intraepithelial cytology was assessed as moderate; that with atypical squamous cells-undetermined significance and "all cytology" was assessed as high. … Human papillomavirus genotyping discriminated risk of CIN 3 or worse to a clinically significant degree, regardless of cytology result” (Bonde et al., 2020).
Between 2010 and 2019, Pry et al. (2021) reviewed 204,225 results from 183,165 women across 11 government health facilities in Lusaka, Zambia, as part of the Cervical Cancer Prevention Program in Zambia (CCPPZ). By examining precancerous lesions via visual inspection with acetic acid and digital cervicography (VIAC), they were “able to show that the highest odds for screening positive are among women aged 20 – 29 years” and that “women in the 30 – 39 years age group had the highest proportion of positive screening results (11·3%) among those with age recorded”; interestingly, however, “Women who were HIV-positive and younger than 20 years had more than three times the predictive probability (18·4, 95% CI 9·56 – 27·32) for being positive compared with women who were HIV-negative in the same age group (predictive probability 5·5%, 95% CI 3·2 – 7·8)” (Pry et al., 2021). But while the high proportion of the screen positivity in women younger than 20 years old may suggest “that women with HIV have earlier disease progression” and “that these women should be engaged in screening at a younger age,” these data could be the result of “some misalignment between screening test positivity and neoplastic lesions, as visually, cervicitis and other benign cervical lesions could be mistaken for pre-cancerous disease” or even simply the inherent weaknesses in the test accuracy of the VIAC method (“sensitivity from 25% [95% CI 7 – 59] to 82% [66 – 95] and specificity from 74% [64 – 82] to 83% [77 – 87]”), warranting further examination (Pry et al., 2021).
Many guidelines call for the cessation of cervical cancer screening after the age of 65; however, Dilley et al. (2021) argues for a re-evaluation of recommendations of this ilk, given that 20% of new cervical cancers occur in this group. Moreover, elderly women are not only more likely to be diagnosed with late-stage cancer, but also receive commensurately worse outcomes and higher mortality rates. The authors point to the use of theoretical modelling and expert opinion as leading drivers of misconceptions about cervical screening harm in older women, specifying that while many of the models seek to minimize the harms and costs associated with increased colposcopies, they are remiss in their consideration of the costs and benefits of “the treatment of advanced cancer, such as cold knife conization, radical hysterectomy, pelvic radiation therapy and chemotherapy” and in their interpretation of exiguous data on the benefits and harms of screening after 65,. This is in addition to the inadequate accounting of incidence across different races, especially in the black population. Furthermore, though the existing guidelines suggest that “the guidelines account for the importance of adequate prior screening before cessation of screening, as the majority of cervical cancer cases are diagnosed in women who have not been adequately screened”, the authors counter that “studies have shown that only 25% – 50% of women diagnosed with cervical cancer had “adequate prior screening” before their cancer diagnosis, which will only be further exacerbated as the population continues to age (Dilley et al., 2021).
U.S. Preventive Services Task Force (USPSTF)
The USPSTF updated their recommendations in 2018. The recommendations are outlined in the table below. The USPSTF did change the recommendation concerning women aged 30 – 65 to now include the possibility of high-risk HPV testing alone once every 5 years as a screening. They still allow the possibility of co-testing every 5 years or for Pap testing alone every 3 years.
The USPSTF notes certain risk factors that may increase the risk of cervical cancer, such as “HIV infection, a compromised immune system, in utero exposure to diethylstilbestrol, and previous treatment of a high-grade precancerous lesion or cervical cancer.” Cytology, primary testing for high-risk HPV alone, or both methods simultaneously may detect the high-risk lesions that are precursors to cervical cancer (USPSTF, 2018).
USPSTF Summary of Recommendations and Evidence (USPSTF, 2018)
|
Population |
Recommendation |
Grade |
|
Women 21 to 29 |
Screen for cervical cancer every 3 years with cytology alone. For women 30-65 years, screen for cervical cancer every 3 years with cytology alone, every 5 years with high-risk (hr) HPV testing alone, or every 5 years with co-testing. |
The USPSTF recommends the service. There is high certainty that the net benefit is substantial. Offer or provide this service. Grade A |
|
Women younger than 21, older than 65, who have had adequate prior screening, or who have had had a hysterectomy |
Do not screen for cervical cancer. |
The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. Discourage the use of this service. Grade D |
In 2017, “The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of performing screening pelvic examinations in asymptomatic, nonpregnant adult women. (I statement) This statement does not apply to specific disorders for which the USPSTF already recommends screening (ie, screening for cervical cancer with a Papanicolaou smear, screening for gonorrhea and chlamydia).”
National Comprehensive Cancer Network (NCCN)
Regarding the diagnosis and workup for cervical cancer, the NCCN states that “The earliest stages of cervical carcinoma may be asymptomatic or associated with a watery vaginal discharge and postcoital bleeding or intermittent spotting. Often these early symptoms are not recognized by the patient. Because of the accessibility of the uterine cervix, cervical cytology or Papanicolaou (Pap) smears and cervical biopsies can usually result in an accurate diagnosis. Cone biopsy (i.e., conization) is recommended if the cervical biopsy is inadequate to define invasiveness or if accurate assessment of microinvasive disease is required. However, cervical cytologic screening methods are less useful for diagnosing adenocarcinoma, because adenocarcinoma in situ affects areas of the cervix that are harder to sample (i.e., endocervical canal)” and that “Workup for these patients with suspicious symptoms includes history and physical examination, complete blood count (CBC; including platelets), and liver and renal function tests” (NCCN, 2021).
The NCCN also remarked that “Persistent human papillomavirus (HPV) infection is the most important factor in the development of cervical cancer. The incidence of cervical cancer appears to be related to the prevalence of HPV in the population. … Screening methods using HPV testing may increase detection of adenocarcinoma,” adducing that “In developed countries, the substantial decline in incidence and mortality of squamous cell carcinoma of the cervix is presumed to be the result of effective screening, although racial, ethnic, and geographic disparities exist” (NCCN, 2021). As such, the NCCN lists chronic, persistent HPV infection along with persistently abnormal Pap smear tests as criteria to be considered for women contemplating hysterectomy.
National Cancer Institute (NCI)
Concerning the use of Pap testing in screening, the NCI recommends: “Based on solid evidence, regular screening for cervical cancer with the Pap test in an appropriate population of women reduces mortality from cervical cancer. The benefits of screening women younger than 21 years are small because of the low prevalence of lesions that will progress to invasive cancer. Screening is not beneficial in women older than 65 years if they have had a recent history of negative test results. … Based on solid evidence, regular screening with the Pap test leads to additional diagnostic procedures (e.g., colposcopy) and treatment for low-grade squamous intraepithelial lesions (LSILs), with long-term consequences for fertility and pregnancy. These harms are greatest for younger women, who have a higher prevalence of LSILs, lesions that often regress without treatment. Harms are also increased in younger women because they have a higher rate of false-positive results” (NCI, 2021).
Concerning the use of HPV DNA testing, the NCI states: “Based on solid evidence, screening with the HPV DNA or HPV RNA test detects high-grade cervical dysplasia, a precursor lesion for cervical cancer. Additional clinical trials show that HPV testing is superior to other cervical cancer screening strategies. In April 2014, the U.S. Food and Drug Administration approved an HPV DNA test that can be used alone for the primary screening of cervical cancer risk in women aged 25 years and older. … Based on solid evidence, HPV testing identifies numerous infections that will not lead to cervical dysplasia or cervical cancer. This is especially true in women younger than 30 years, in whom rates of HPV infection may be higher (NCI, 2021).”
Concerning cotesting, they recommend: “Based on solid evidence, screening every 5 years with the Pap test and the HPV DNA test (cotesting) in women aged 30 years and older is more sensitive in detecting cervical abnormalities, compared with the Pap test alone. Screening with the Pap test and HPV DNA test reduces the incidence of cervical cancer. … Based on solid evidence, HPV and Pap cotesting is associated with more false-positives than is the Pap test alone. Abnormal test results can lead to more frequent testing and invasive diagnostic procedures (NCI, 2021).”
American Cancer Society (ACS)
The American Cancer Society updated their guidelines for cervical cancer screening for individuals at average risk in 2020. Their recommendations are summarized below:
(Adapted from Table 2 of (Fontham et al., 2020), Comparison of Current and Previous American Cancer Society (ACS) Guidelines for Cervical Cancer Screening)
| Population | 2020 ACS Recommendation |
| Age 21 – 24 | No screening |
| Age 25 – 29 |
HPV test every 5 years (preferred) |
|
Age 30 – 65
|
HPV test every 5 years (preferred) |
| Age 65 and older | No screening if a series of prior tests were normal |
(Fontham et al., 2020).
Choosing Wisely and the American Society for Colposcopy and Cervical Pathology (ASCCP)
The ASCCP recommends: “Don’t perform cervical cytology (Pap tests) or HPV screening in patients under age 21 who have a normal immune system. Cervical cancer is rare in adolescents and screening does not appear to lower that risk. Screening adolescents for cervical cancer exposes them to the potential harms of tests, biopsies, and procedures, without proven benefit” (ASCCP, 2019).
The ASCCP also recommends against screening for low-risk HPV types (ASCCP, 2017).
In 2019, the ASCCP also published guidelines for cervical cancer screening in immunosuppressed women without an HIV infection. The following table was provided by Moscicki et al. (2019):
Table 3. Summary of Cervical Cancer Screening Recommendations for Non-HIV Immunocompromised Women
|
Risk group category |
Recommendation |
|
Solid organ transplant |
|
|
Allogeneic hematopoietic stem cell transplant |
|
|
Inflammatory bowel disease on immunosuppressant treatments |
|
|
Inflammatory bowel disease not on immunosuppressant treatment |
|
|
Systemic lupus erythematosus and rheumatoid arthritis on immunosuppressant treatments |
|
|
Rheumatoid arthritis not on immunosuppressive treatments |
|
|
Type 1 diabetes mellitus |
|
In 2021, the ASCCP updated and expounded upon their guidelines titled “Five Things Physicians and Patients Should Question” through Choosing Wisely. The relevant recommendations about cervical cytology and HPV screening are reported below:
- “Don’t perform vaginal cytology (Pap test) or HPV screening in patients who had hysterectomy (with removal of the cervix) and have no history of high-grade cervical dysplasia (CIN 2+) or cancer.”
- “Don’t perform cervical cytology (Pap tests) or HPV screening in patients under age 21 who have a normal immune system.”
- “Don’t order screening tests for low-risk HPV types.”
- “Don’t perform annual cervical cancer screening (Pap test, HPV screening or cotesting) on asymptomatic patients who have had adequate and normal screening results and have a normal immune system” (ASCCP, 2021).
Society of Gynecologic Oncology, American Society for Colposcopy and Cervical Pathology, American College of Obstetricians and Gynecologists, American Cancer Society, American Society of Cytopathology, College of American Pathologists, and the American Society for Clinical Pathology
Since the 2011 joint guidelines issued by the American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening concerning cervical cancer screening, additional reports regarding the use of primary hrHPV testing so that representatives from the Society of Gynecologic Oncology, American Society for Colposcopy and Cervical Pathology, American College of Obstetricians and Gynecologists, American Cancer Society, American Society of Cytopathology, College of American Pathologists, and the American Society for Clinical Pathology convened to issue interim clinical guidance in 2015. In the 2011 statement, primary hrHPV testing was not recommended. The 2015 recommendations include:
- “Because of equivalent or superior effectiveness, primary hrHPV screening can be considered as an alternative to current U.S. cytology-based cervical cancer screening methods. Cytology alone and cotesting remain the screening options specifically recommended in major guidelines.”
- “A negative hrHPV test provides greater reassurance of low CIN3+ risk than a negative cytology result.”
- “Rescreening after a negative primary hrHPV screen should occur no sooner than every 3 years.”
- “Primary hrHPV screening should not be initiated prior to 25 years of age.”
Moreover, they give the following screening algorithm:
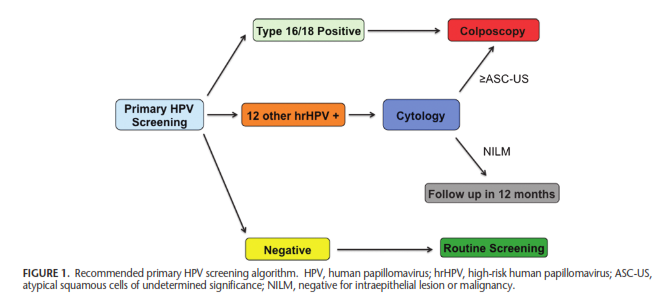
American College of Obstetricians and Gynecologists (ACOG)
In April 2021, the ACOG released a statement withdrawing and replacing the Practice Bulletin No.168 on cervical cancer screening, stating that it will be joining the ASCCP and the SGO “in endorsing the U.S. Preventive Services Task Force (USPSTF) cervical cancer screening recommendations, which replace ACOG Practice Bulletin No.168, Cervical Cancer Screening and Prevention, as well as the 2012 ASCCP cervical cancer screening guidelines” (ACOG, 2021).
In October 2020, the ACOG released “Updated Guidelines for Management of Cervical Cancer Screening Abnormalities”. These new consensus guidelines are based on risk to determine screening, surveillance, colposcopy, or treatment later in life (ACOG, 2020). In relation to screening, the updated management guidelines state:
- “Recommendations are based on risk, not results.
- Recommendations of colposcopy, treatment, or surveillance will be based on a patient's risk of CIN 3+ determined by a combination of current results and past history (including unknown history). The same current test results may yield different management recommendations depending on the history of recent past test results.
- Colposcopy can be deferred for certain patients.
- Repeat human papillomavirus (HPV) testing or cotesting at 1 year is recommended for patients with minor screening abnormalities indicating HPV infection with low risk of underlying CIN 3+ (eg, HPV-positive, low-grade cytologic abnormalities after a documented negative screening HPV test or cotest).
- All positive primary HPV screening tests, regardless of genotype, should have additional reflex triage testing performed from the same laboratory specimen (e.g., reflex cytology).
- Additional testing from the same laboratory specimen is recommended because the findings may inform colposcopy practice. For example, those HPV-16 positive HSIL cytology qualify for expedited treatment.
- HPV 16 or 18 infections have the highest risk for CIN 3 and occult cancer, so additional evaluation (e.g., colposcopy with biopsy) is necessary even when cytology results are negative.
- If HPV 16 or 18 testing is positive, and additional laboratory testing of the same sample is not feasible, the patient should proceed directly to colposcopy.
- Continued surveillance with HPV testing or cotesting at 3-year intervals for at least 25 years is recommended after treatment and initial posttreatment management of histologic HSIL, CIN 2, CIN 3, or AIS. Continued surveillance at 3-year intervals beyond 25 years is acceptable for as long as the patient's life expectancy and ability to be screened are not significantly compromised by serious health issues.
- New evidence indicates that risk remains elevated for at least 25 years, with no evidence that treated patients ever return to risk levels compatible with 5-year intervals.
- Surveillance with cytology alone is acceptable only if testing with HPV or cotesting is not feasible. Cytology is less sensitive than HPV testing for detection of precancer and is therefore recommended more often. Cytology is recommended at 6-month intervals when HPV testing or cotesting is recommended annually. Cytology is recommended annually when 3-year intervals are recommended for HPV or cotesting.
- Human papilloma virus assays that are Food and Drug Administration (FDA)-approved for screening should be used for management according to their regulatory approval in the United States. (Note: all HPV testing in [the guidelines] refers to testing for high-risk HPV types only).
- For all management indications, HPV mRNA and HPV DNA tests without FDA approval for primary screening alone should only be used as a cotest with cytology, unless sufficient, rigorous data are available to support use of these particular tests in management” (ACOG, 2020).
Table of Terminology
|
Term |
Definition |
|
ACOG |
American College of Obstetricians and Gynecologists |
|
ACS |
American Cancer Society |
|
AIS |
Adenocarcinoma in situ |
|
ASCCP |
American Society for Colposcopy and Cervical Pathology |
|
ASC-H |
Atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion |
|
ASCP |
American Society of Clinical pathology |
|
ASCUS |
Atypical squamous cells of undetermined significance |
|
CIN (2, 3, 3+) |
Cervical intraepithelial neoplasia (Grade 2, 3, 3+) |
|
CLIA ’88 |
Clinical Laboratory Improvement Amendments of 1988 |
|
CMS |
Centers for Medicare & Medicaid Services |
|
DES |
Diethylstilbestrol |
|
DNA |
Deoxyribonucleic acid |
|
FDA |
Food and Drug Administration |
|
GvHD |
Graft versus/against the host disease |
|
HGSIL/HSIL |
High-grade squamous intraepithelial lesion |
|
HIV |
Human immunodeficiency virus |
|
HPV |
Human papillomavirus infection |
|
HSCT |
Hematopoietic stem cell transplantation |
|
IPD |
Individual participant data |
|
JAMA |
Journal of the American Medical Association |
|
LDTs |
Laboratory developed tests |
|
LGSIL/LSIL |
Low-grade squamous intraepithelial lesion |
| mRNA |
Messenger ribonucleic acid |
|
NAAT |
Nucleic acid amplification test |
|
NCCN |
National Comprehensive Cancer Network |
|
NCI |
National Cancer Institute |
|
Pap |
Papanicolaou |
|
PCR |
Polymerase chain reaction |
|
RNA |
Ribonucleic acid |
|
SGO |
Society of Gynecologic Oncology |
|
STIs |
Sexually transmitted infections |
|
USPSTF |
U.S. Preventive Services Task Force |
|
VIAC |
Visual inspection with acetic acid and digital cervicography |
References:
- ACOG. (2020, October 9). Updated Guidelines for Management of Cervical Cancer Screening Abnormalities. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/10/updated-guidelines-for-management-of-cervical-cancer-screening-abnormalities
- ACOG. (2021, April 12). Updated Cervical Cancer Screening Guidelines. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines
- ACS. (2021, January 12). Key Statistics for Cervical Cancer. American Cancer Society, Inc. Retrieved 08/15/2018 from https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html
- ASCCP. (2017, February 14). Don’t order screening tests for low-risk HPV types. ABIM. http://www.choosingwisely.org/clinician-lists/asccp-screening-tests-for-low-risk-hpv-types/
- ASCCP. (2019, October 3). Don’t perform cervical cytology (Pap tests) or HPV screening in patients under age 21 who have a normal immune system. ABIM. http://www.choosingwisely.org/clinician-lists/asccp-pap-tests-or-hpv-screening-in-women-under-21/
- ASCCP. (2021, October 1, 2021). Five Things Physicians and Patients Should Question. Choosing Wisely. https://www.choosingwisely.org/societies/asccp/
- Bonde, J. H., Sandri, M. T., Gary, D. S., & Andrews, J. C. (2020). Clinical Utility of Human Papillomavirus Genotyping in Cervical Cancer Screening: A Systematic Review. J Low Genit Tract Dis, 24(1), 1-13. https://doi.org/10.1097/lgt.0000000000000494
- Chen, H. C., Schiffman, M., Lin, C. Y., Pan, M. H., You, S. L., Chuang, L. C., Hsieh, C. Y., Liaw, K. L., Hsing, A. W., & Chen, C. J. (2011). Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst, 103(18), 1387-1396. https://doi.org/10.1093/jnci/djr283
- Dahlstrom, L. A., Ylitalo, N., Sundstrom, K., Palmgren, J., Ploner, A., Eloranta, S., Sanjeevi, C. B., Andersson, S., Rohan, T., Dillner, J., Adami, H. O., & Sparen, P. (2010). Prospective study of human papillomavirus and risk of cervical adenocarcinoma. Int J Cancer, 127(8), 1923-1930. https://doi.org/10.1002/ijc.25408
- Dilley, S., Huh, W., Blechter, B., & Rositch, A. F. (2021). It's time to re-evaluate cervical Cancer screening after age 65. Gynecol Oncol, 162(1), 200-202. https://doi.org/10.1016/j.ygyno.2021.04.027
- EAU. (2021). Prostate Cancer. https://uroweb.org/guideline/prostate-cancer/#5
- FDA. (2018a). BD ONCLARITY HPV ASSAY. https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pma&id=391601
- FDA. (2018b, 07/09/2018). BD ONCLARITY HPV ASSAY. U.S. Food & Drug Administration. Retrieved 07/11/2018 from https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pma&id=391601
- FDA. (2018c, 08/06/2018). PMA Monthly approvals from 7/1/2018 to 7/31/2018. Food and Drug Agency. Retrieved 08/21/2018 from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?ID=409848
- FDA. (2019). Devices@FDA. U.S. Food & Drug Administration. https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm
- FDA. (2020). Cobas HPV For Use On The Cobas 6800/8800 Systems. https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pma&id=448383
- Feldman, S., & Crum, C. (2022, May 2, 2022). Cervical cancer screening tests: Techniques for cervical cytology and human papillomavirus testing. https://www.uptodate.com/contents/cervical-cancer-screening-tests-techniques-for-cervical-cytology-and-human-papillomavirus-testing
- Feldman, S., Goodman, A., & Peipert, J. (2022, March 17). Screening for cervical cancer in resource-rich settings. https://www.uptodate.com/contents/screening-for-cervical-cancer-in-resource-rich-settings
- Fontham, E. T. H., Wolf, A. M. D., Church, T. R., Etzioni, R., Flowers, C. R., Herzig, A., Guerra, C. E., Oeffinger, K. C., Shih, Y. T., Walter, L. C., Kim, J. J., Andrews, K. S., DeSantis, C. E., Fedewa, S. A., Manassaram-Baptiste, D., Saslow, D., Wender, R. C., & Smith, R. A. (2020). Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin, 70(5), 321-346. https://doi.org/10.3322/caac.21628
- Huh, W. K., Ault, K. A., Chelmow, D., Davey, D. D., Goulart, R. A., Garcia, F. A., Kinney, W. K., Massad, L. S., Mayeaux, E. J., Saslow, D., Schiffman, M., Wentzensen, N., Lawson, H. W., & Einstein, M. H. (2015). Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. J Low Genit Tract Dis, 19(2), 91-96. https://doi.org/10.1097/lgt.0000000000000103
- Marchand, L., Mundt, M., Klein, G., & Agarwal, S. C. (2005). Optimal collection technique and devices for a quality pap smear. Wmj, 104(6), 51-55.
- Massad, L. S. (2018). Replacing the Pap Test With Screening Based on Human Papillomavirus Assays. Jama, 320(1), 35-37. https://doi.org/10.1001/jama.2018.7911
- Melnikow, J., Henderson, J. T., Burda, B. U., Senger, C. A., Durbin, S., & Weyrich, M. S. (2018). Screening for Cervical Cancer With High-Risk Human Papillomavirus Testing: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama, 320(7), 687-705. https://doi.org/10.1001/jama.2018.10400
- Mendez, K., Romaguera, J., Ortiz, A. P., Lopez, M., Steinau, M., & Unger, E. R. (2014). Urine-based human papillomavirus DNA testing as a screening tool for cervical cancer in high-risk women. Int J Gynaecol Obstet, 124(2), 151-155. https://doi.org/10.1016/j.ijgo.2013.07.036
- Moscicki, A. B., Flowers, L., Huchko, M. J., Long, M. E., MacLaughlin, K. L., Murphy, J., Spiryda, L. B., & Gold, M. A. (2019). Guidelines for Cervical Cancer Screening in Immunosuppressed Women Without HIV Infection. J Low Genit Tract Dis, 23(2), 87-101. https://doi.org/10.1097/lgt.0000000000000468
- NCCN. (2021, October 26, 2021). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines(R)) - Cervical Cancer Version 1.2022. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
- NCI. (2021, March 24). Cervical Cancer Screening (PDQ®)–Health Professional Version. National Institutes of Health. https://www.cancer.gov/types/cervical/hp/cervical-screening-pdq
- Ogilvie, G. S., van Niekerk, D., Krajden, M., Smith, L. W., Cook, D., Gondara, L., Ceballos, K., Quinlan, D., Lee, M., Martin, R. E., Gentile, L., Peacock, S., Stuart, G. C. E., Franco, E. L., & Coldman, A. J. (2018). Effect of Screening With Primary Cervical HPV Testing vs Cytology Testing on High-grade Cervical Intraepithelial Neoplasia at 48 Months: The HPV FOCAL Randomized Clinical Trial. Jama, 320(1), 43-52. https://doi.org/10.1001/jama.2018.7464
- Pathak, N., Dodds, J., Zamora, J., & Khan, K. (2014). Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. Bmj, 349, g5264. https://doi.org/10.1136/bmj.g5264
- Pry, J. M., Manasyan, A., Kapambwe, S., Taghavi, K., Duran-Frigola, M., Mwanahamuntu, M., Sikazwe, I., Matambo, J., Mubita, J., Lishimpi, K., Malama, K., & Bolton Moore, C. (2021). Cervical cancer screening outcomes in Zambia, 2010-19: a cohort study. Lancet Glob Health, 9(6), e832-e840. https://doi.org/10.1016/s2214-109x(21)00062-0
- Rice, S. L., Editor. (2018, August 2018). Cobas HPV test approved for first-line screening using SurePath preservative fluid. CAP Today.
- Sabeena, S., Kuriakose, S., Binesh, D., Abdulmajeed, J., Dsouza, G., Ramachandran, A., Vijaykumar, B., Aswathyraj, S., Devadiga, S., Ravishankar, N., & Arunkumar, G. (2019). The Utility of Urine-Based Sampling for Cervical Cancer Screening in Low-Resource Settings. Asian Pac J Cancer Prev, 20(8), 2409-2413. https://doi.org/10.31557/apjcp.2019.20.8.2409
- Sasieni, P., Castanon, A., & Cuzick, J. (2009). Screening and adenocarcinoma of the cervix. Int J Cancer, 125(3), 525-529. https://doi.org/10.1002/ijc.24410
- USPSTF. (2018). Screening for Cervical Cancer: US Preventive Services Task Force Recommendation StatementUSPSTF Recommendation: Screening for Cervical CancerUSPSTF Recommendation: Screening for Cervical Cancer. Jama, 320(7), 674-686. https://doi.org/10.1001/jama.2018.10897
Coding Section
|
Code |
Number |
Description |
|
CPT |
87623 |
Infectious agent detection by nucleic acid (DNA or RNA); Human Papillomavirus (HPV), low-risk types (e.g., 6, 11, 42, 43, 44) |
|
|
87624 |
Infectious agent detection by nucleic acid (DNA or RNA); Human Papillomavirus (HPV), high-risk types (e.g., 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) |
|
|
87625 |
Infectious agent detection by nucleic acid (DNA or RNA); Human Papillomavirus (HPV), types 16 and 18 only, includes type 45, if performed |
|
|
88141 |
Cytopathology, cervical or vaginal (any reporting system), requiring interpretation by physician |
|
|
88142 |
Cytopathology, cervical or vaginal (any reporting system), collected in preservative fluid, automated thin layer preparation; manual screening under physician supervision |
|
|
88143 |
Cytopathology, cervical or vaginal (any reporting system), collected in preservative fluid, automated thin layer preparation; with manual screening and rescreening under physician supervision |
|
|
88147 |
Cytopathology smears, cervical or vaginal; screening by automated system under physician supervision |
|
|
88148 |
Cytopathology smears, cervical or vaginal; screening by automated system with manual rescreening under physician supervision |
|
|
88150 |
Cytopathology, slides, cervical or vaginal; manual screening under physician supervision |
|
|
88152 |
Cytopathology, slides, cervical or vaginal; with manual screening and computer-assisted rescreening under physician supervision |
|
|
88153 |
Cytopathology, slides, cervical or vaginal; with manual screening and rescreening under physician supervision |
|
|
88164 |
Cytopathology, slides, cervical or vaginal (the bethesda system); manual screening under physician supervision |
|
|
88165 |
Cytopathology, slides, cervical or vaginal (the bethesda system); with manual screening and rescreening under physician supervision |
|
|
88166 |
Cytopathology, slides, cervical or vaginal (the bethesda system); with manual screening and computer-assisted rescreening under physician supervision |
|
|
88167 |
Cytopathology, slides, cervical or vaginal (the bethesda system); with manual screening and computer-assisted rescreening using cell selection and review under physician supervision |
|
|
88174 |
Cytopathology, cervical or vaginal (any reporting system), collected in preservative fluid, automated thin layer preparation; screening by automated system, under physician supervision |
|
|
88175 |
Cytopathology, cervical or vaginal (any reporting system), collected in preservative fluid, automated thin layer preparation; with screening by automated system and manual rescreening or review, under physician supervision |
|
|
0500T |
Infectious agent detection by nucleic acid (DNA or RNA), Human Papillomavirus (HPV) for five or more separately reported high-risk HPV types (e.g., 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) (i.e., genotyping) |
|
|
G0123 |
Screening cytopathology, cervical or vaginal (any reporting system), collected in preservative fluid, automated thin layer preparation, screening by cytotechnologist under physician supervision |
|
|
G0124 |
Screening cytopathology, cervical or vaginal (any reporting system), collected in preservative fluid, automated thin layer preparation, requiring interpretation by physician |
|
|
G0141 |
Screening cytopathology smears, cervical or vaginal, performed by automated system, with manual rescreening, requiring interpretation by physician |
|
|
G0143 |
Screening cytopathology, cervical or vaginal (any reporting system), collected in preservative fluid, automated thin layer preparation, with manual screening and rescreening by cytotechnologist under physician supervision |
|
|
G0144 |
Screening cytopathology, cervical or vaginal (any reporting system), collected in preservative fluid, automated thin layer preparation, with screening by automated system, under physician supervision |
|
|
G0145 |
Screening cytopathology, cervical or vaginal (any reporting system), collected in preservative fluid, automated thin layer preparation, with screening by automated system and manual rescreening under physician supervision |
|
|
G0147 |
Screening cytopathology smears, cervical or vaginal, performed by automated system under physician supervision |
|
|
G0148 |
Screening cytopathology smears, cervical or vaginal, performed by automated system with manual rescreening |
|
|
G0476 |
Infectious agent detection by nucleic acid (DNA or RNA); human papillomavirus (HPV), high-risk types (e.g., 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) for cervical cancer screening, must be performed in addition to pap test |
|
|
P3000 |
Screening Papanicolaou smear, cervical or vaginal, up to three smears, by technician under physician supervision |
|
|
P3001 |
Screening Papanicolaou smear, cervical or vaginal, up to three smears, requiring interpretation by physician |
|
|
Q0091 |
Screening Papanicolaou smear; obtaining, preparing and conveyance of cervical or vaginal smear to laboratory |
|
ICD-10 |
N84.1 |
Polyp of cervix uteri |
|
|
N88.8 |
Other specified noninflammatory disorders of cervix uteri |
|
|
N88.9 |
Noninflammatory disorder of cervix uteri, unspecified |
|
|
N93.0 |
Postcoital and contact bleeding |
|
|
N94.10 |
Unspecified dyspareunia |
|
|
Z91.419 |
Personal history of unspecified adult abuse |
|
|
N95.0 |
Postmenopausal bleeding |
|
|
R87.5 |
Abnormal microbiological findings in specimens from female genital organs |
|
|
Z08. |
Encounter for follow-up examination after completed treatment for malignant neoplasm |
|
|
Z09 |
Encounter for follow-up examination after completed treatment for conditions other than malignant neoplasm |
|
|
Z77.29 |
Contact with and (suspected) exposure to other hazardous substances |
|
|
Z20.2 |
Contact with and (suspected) exposure to infections with a predominantly sexual mode of transmission |
|
|
Z79.899 |
Other long term (current) drug therapy |
|
|
Z85.40 |
Personal history of malignant carcinoid tumor of rectum |
|
|
Z85.44 |
Personal history of malignant neoplasm of other female genital organs |
|
|
Z86.001 |
Personal history of in-situ neoplasm of cervix uteri |
|
|
Z86.002 |
Personal history of in-situ neoplasm of other and unspecified genital organs |
|
|
Z20.828 |
Contact with and (suspected) exposure to other viral communicable diseases |
|
|
Z86.2 |
Personal history of diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism |
|
|
Z87.412 |
Personal history of vulvar dysplasia |
|
|
Z92.89 |
Personal history of other medical treatment |
|
|
Z77.9 |
Other contact with and (suspected) exposures hazardous to health |
|
|
Z92.21 |
Personal history of antineoplastic chemotherapy |
|
|
Z20.9 |
Contact with and (suspected) exposure to unspecified communicable disease |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, Blue Cross Blue Shield Association technology assessment program (TEC) and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2014 Forward
| 11/09/2022 | Annual review, no change to policy intent. Updating policy for clarity. Adding table of terminology. Updating rationale and references. |
|
10/01/2021 |
Annual review, no change to policy intent. Updating background, rationale, references and coding. |
|
10/01/2020 |
Annual review, adding policy verbiage related to immunocompromised members. Updating coding, description, rationale and references. |
|
10/09/2019 |
Annual review, no change to policy intent. Updating coding. |
|
11/27/2018 |
Annual review, updating medical necessity criteria related to HPV testing. No other changes. |
|
12/7/2017 |
Removed cpt code 88154 per 2018 coding and add cpt code 0500T. No other changes |
|
10/19/2017 |
Annual review, no change to policy intent. |
|
06/19/2017 |
Updated coding section. No other changes made. |
|
05/24/2017 |
Corrected Typo in Policy Guideline section. No change to policy intent. |
|
04/26/2017 |
Updated category to Laboratory. No other changes. |
|
07/18/2016 |
Annual review, review month moved to July from June. Updating policy verbiage for specificity. Updating background and coding. |
|
03/09/2016 |
Interim review, adding the following verbiage: Primary HPV testing (testing for HPV without cytology) is considered investigational as a method of screening for cervical cancer. |
|
06/25/2015 |
Annual review, no change to policy intent. Updated guidelines and added coding. |
|
07/07/2014 |
Annual review. Policy criteria updated for both Pap smear and HPV testing. 2012 ACOG guidelines added to update previous guidelines. |