Diagnostic Testing of Influenza - CAM 134
Description
Influenza is an acute respiratory illness caused by influenza A or B viruses resulting in upper and lower respiratory tract infection, fever, malaise, headache, and weakness. It mainly occurs in outbreaks and epidemics during the winter season, and is associated with increased morbidity and mortality in certain high-risk populations.
Rapid influenza diagnostic tests (RIDTs) refer to clinical laboratory improvement amendments (CLIA) waived immunoassays that can detect influenza viruses during the outpatient visit, giving results in a clinically relevant time period to inform treatment decisions. Besides RIDTs, influenza can be detected using polymerase chain reaction (PCR)-based assays as well as culture testing; however, the former is not often used in initial clinical management due to time constraints. Serologic testing is not used in outpatient settings for diagnosis.
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
- For diagnosis in patients who present in the outpatient setting with signs and symptoms consistent with influenza disease (See Note 1 below) when influenza activity has been documented in the community or geographic area, ONE, but NOT BOTH, of the following is considered MEDICALLY NECESSARY.:
- One single rapid flu test includes either a point-of-contact rapid nucleic acid amplification test (NAAT) or a rapid antigen test; OR
- One single traditional NAAT.
- Viral culture testing for influenza in an outpatient setting is considered NOT MEDICALLY NECESSARY.
- In asymptomatic patients, outpatient influenza testing, including rapid antigen flu tests, rapid NAAT or RT-PCR tests, traditional RT-PCR tests, and viral culture testing, is considered NOT MEDICALLY NECESSARY.
- Serology testing for influenza is considered NOT MEDICALLY NECESSARY under any circumstance.
Note 1:Typical Influenza Signs and Symptoms (CDC, 2020a)
- Fever: A 100.4◦F or higher temperature or feeling feverish/chills AND one or more:
- Cough
- Sore throat
- Headaches and/or body aches
- Difficulty breathing or shortness of breath
- Fatigue
- Runny or stuffy nose
Table of Terminology
|
Term |
Definition |
|
AAEM |
American Academy of Emergency Medicine |
|
AAP |
American Academy of Paediatrics |
|
ATS |
American Thoracic Society |
|
CDC |
Centers for Disease Control and Prevention |
|
CLIA |
Clinical Laboratory Improvement Amendments |
|
DFA/IFA |
Direct or Indirect fluorescent antibody staining |
|
DNA |
Deoxyribonucleic acid |
|
EIA |
Enzyme immunoassay |
|
ELISA |
Enzyme-linked immunosorbent assay |
|
FBC |
Full blood counts |
|
FDA |
Food and Drug Administration |
|
FIA |
Fluorescence immunoassay |
|
ICT |
Immunochromatographic |
|
IDSA |
Infectious Diseases Society of America |
|
IMCA |
Immunochemiluminometric assay |
|
MDCK |
Madin-Darby Canine Kidney |
|
NAAT |
Nucleic acid amplification test |
|
NIBSC |
National Institute for Biological Standards and Control |
|
NIH |
National Institute of Health |
|
NPS |
Nasopharyngeal Swab |
|
NPV |
Negative predictive value |
|
PCR |
Polymerase chain reaction |
|
POC |
Point-of-care |
|
PPV |
Positive predictive value |
|
RAD |
Rapid antigen diagnostic |
|
RIDTs |
Rapid influenza diagnostic tests |
|
RSV |
Respiratory syncytial virus |
|
RT-PCR |
Reverse-transcriptase polymerase chain reaction |
Rationale
The influenza virus causes seasonal epidemics that result in severe illnesses and death every year. Influenza characteristically begins with the abrupt onset of fever, headache, myalgia, and malaise (Dolin, 1976; Kilbourne & Loge, 1950; Loeb et al., 2012; Nicholson, 1992), accompanied by manifestations of respiratory tract illness, such as nonproductive cough, sore throat, and nasal discharge (Dolin, 2022b).
High titers of influenza virus are often present in respiratory secretions of infected persons. Influenza is transmitted primarily via respiratory droplets produced from sneezing and coughing (Brankston et al., 2007; Dolin, 2022b; Mubareka et al., 2009) which requires close contact with an infected individual. The typical incubation period for influenza is one to four days (average two days) (CDC, 2017; Cox & Subbarao, 1999). The serial interval among household contacts is three to four days (Cowling et al., 2010). When initiated promptly (within the first 24 to 30 hours), antiviral therapy can shorten the duration of influenza symptoms by approximately one-half to three days (Cooper et al., 2003; Dobson et al., 2015; Hayden et al., 1997; Heneghan et al., 2014; Jefferson et al., 2014; Nicholson et al., 2000; Zachary, 2022).
In certain circumstances, the diagnosis of influenza can be made clinically, such as during an outbreak. At other times, it is important to establish the diagnosis using laboratory testing. Viral diagnostic test options include rapid antigen tests, immunofluorescence assays, and reverse-transcriptase polymerase chain reaction (RT-PCR)-based testing (CDC, 2017). Among these, RT-PCR is the most sensitive and specific (Dolin, 2022a). Rapid influenza antigen tests are immunoassays that can identify influenza A and B viral nucleoprotein antigens in respiratory specimens (CDC, 2017) which yield qualitative results in approximately 15 minutes or less. However, they have much lower sensitivity (CDC, 2017; Harper et al., 2009; Hurt et al., 2007; Ikenaga et al., 2008). A recent meta-analysis found that the sensitivity of these immunoassays was 62.3 percent and the specificity was 98.2 percent (Chartrand et al., 2012). Furthermore, detectable viral shedding in respiratory secretions peaks at 24 to 48 hours of illness and then rapidly declines (Dolin, 2022a).
A decision analysis by Sintchenko et al. (2002) concluded that treatment based on rapid diagnostic testing results was appropriate first over empirical antiviral treatment, except during influenza epidemics. When the probability of a case being due to influenza reached 42 percent, the two strategies were equivalent. Further, a separate meta-analysis found that rapid diagnostic testing did not add to the overall cost-effectiveness of treatment if the probability of influenza was greater than 25 to 30 percent (Call et al., 2005; Dolin, 2022a).
Analytical Validity
Viral culture is a gold standard for influenza diagnosis, but it is very time-consuming with an average 7-day turnaround time; on the other hand, real-time RT-PCR and shell vial (SV) testing require only an average of 4 hours and 48 hours, respectively. A study by Lopez Roa et al. (2011) compared real-time RT-PCR and SV testing against conventional cell culture to detect pandemic influenza A H1N1. The sensitivity of real-time RT-PCR as compared to viral culture testing was 96.5%, and SV had a sensitivity of 73.3% and 65.1%, depending on the use of either A549 cells or Madin-Darby Canine Kidney (MDCK) cells, respectively. The authors conclude, “Real-time RT-PCR displayed high sensitivity and specificity for the detection of influenza A H1N1 in adult patients when compared with conventional techniques” (Lopez Roa et al., 2011).
Clinical Validity and Utility
In 2017, Yoon et al. (2017) investigated the use of saliva specimens for detecting influenza A and B using RIDTs. Both saliva and nasopharyngeal swab (NPS) samples were analyzed from 385 patients; each sample was assayed using four different RIDTs — the Sofia Influenza A+B Fluorescence Immunoassay, ichroma TRIAS Influenza A+B, SD Bioline Influenza Ag, and BinaxNOW Influenza A/B antigen kit — as well as real-time RT-PCR. Using real-time RT-PCR as a standard, 31.2% of the patients tested positive for influenza A and 7.5% for influenza B. All four RIDTS had “slightly higher” diagnostic sensitivity in NPS samples than saliva samples; however, both Sofia and ichroma “were significantly superior to those of the other conventional influenza RIDTs with both types of sample” (Yoon et al., 2017). The authors note that the sensitivity of diagnosis improves if both saliva and NPS testing is performed (from 10% to 13% and from 10.3% to 17.2% for A and B, respectively). The researchers conclude, “This study demonstrates that saliva is a useful specimen for influenza detection, and that the combination of saliva and NPS could improve the sensitivities of influenza RIDTs” (Yoon et al., 2017).
Ryu et al. (2016) investigated the efficacy of using instrument-based digital readout systems with RIDTs. In their 2016 paper, the authors included 314 NPS samples from patients with suspected influenza and tested each sample with the Sofia Influenza A+B Fluorescence Immunoassay and BD Veritor System Flu A+B, which use instrument-based digital readout systems, as well as the SD Bioline assay (a traditional immunochromatographic assay) and PCR, the standard. Relative to the RT-PCR standard, for influenza A, the sensitivities for the Sofia, BD Veritor, and SD Bioline assays were 74.2%, 73.0%, and 53.9%, respectively; likewise, for influenza B, the sensitivities, respectively, were 82.5%, 72.8%, and 71.0%. All RIDTS show 100% specificities for both subtypes A and B. The authors conclude, “Digital-based readout systems for the detection of the influenza virus can be applied for more sensitive diagnosis in clinical settings than conventional [RIDTs]” (Ryu et al., 2016). Similar research was performed in 2018 on NPS using RIDTs with digital readout systems — Sofia and Veritor as before along with BUDDI — as compared to standard RT-PCR and the SD Bioline immunochromatographic assay (n = 218). The four RIDTs were also tested with diluted solutions from the National Institute for Biological Standards and Control (NIBSC) to probe lower detection limits for each testing method. Again, the digital-based assays exhibited higher sensitivity for influenza. “Sofia showed the highest sensitivity for influenza A and B detection. BUDDI and Veritor showed higher detection sensitivity than a conventional RIDT for influenza A detection. Further study is needed to compare the test performance of RIDTs according to specific, prevalent influenza subtypes” (Ryu et al., 2018).
Another study compared the Alere iNAT, a rapid isothermal nucleic acid amplification assay, to the Sofia Influenza A+B and the BinaxNOW Influenza A&B immunochromatographic (ICT) assay. Using RT-PCR as the standard for 202 NPS samples, the “Alere iNAT detected 75% of those positive by RT-PCR, versus 33.3% and 25.0% for Sofia and BinaxNOW, respectively. The specificity of Alere iNAT was 100% for influenza A and 99% for influenza B” (Hazelton et al., 2015). BinaxNOW also had a sensitivity of only 69% for influenza as compared to RT-PCR in another study of 520 NPS from children under the age of 5 (Moesker et al., 2016).
Young et al. (2017) investigated the accuracy of using point-of-care (POC) nucleic acid amplification test (NAAT)-based assays on NPS as compared to the US Food and Drug Administration (FDA)-cleared in vitro PCR test, GenMark Dx Respiratory Viral Panel. Their study consisted of 87 NPS samples from adults. As compared to the RT-PCR gold standard, the cobas Liat Influenza A/B POC test had an overall sensitivity and specificity of 97.9% and 97.5%, respectively, whereas the Alere i Influenza A&B POC test’s sensitivity was only 63.8% with a specificity of 97.5% (Young et al., 2017). Taken together, the authors conclude that “the cobas Influenza A/B assay demonstrated performance equivalent to laboratory-based PCR, and could replace rapid antigen tests” (Young et al., 2017). These results are corroborated by another study that measured the specificity of the cobas POC assay as 100% for influenza A/B with a sensitivity of 96% for influenza A and 100% for influenza B (Melchers et al., 2017). Further, a third study reported a 6.5% invalid rate (as defined by as a failure on a first-run assay) by the cobas POC assay; however, “the sensitivities and specificities for all assays [cobas, Xpert Xpress Flu/RSV, and Aries Flu A/B & RSV] were 96.0 to 100.0% and 99.3 to 100% for all three viruses [influenza A, influenza B, and respiratory syncytial virus]” (Ling et al., 2018).
Antoniol et al. (2018) aimed to evaluate the usage of rapid influenza diagnostic tests (RIDTs) in adults, particularly the OSOM® Ultra Flu A&B on viral strains of influenza A/B in the emergency department. The diagnostic evaluation of this test was compared against the Xpert® Flu PCR test. The PCR test had a sensitivity of 98.4%, specificity of 99.7%, positive predictive value (PPV) of 99.2% and a negative predictive value (NPV) of 99.5% , whereas the OSOM® Ultra Flu A&B RIDT had a sensitivity of 95.1%, specificity of 98.4%, positive predictive value of 95.1%, and negative predictive value of 98.4%. However, “there was no difference in test performance between influenza A and B virus nor between the influenza A subtypes,” thereby solidifying the use of both the PCR and RIDT in diagnosing influenza strains in adult and elderly patients (Antoniol et al., 2018).
Lee et al. (2019) conducted a systematic review and meta-analysis on point-of-care tests (POCTs) for influenza in ambulatory care settings. After screening, seven randomized studies and six non-randomized studies from studies mostly from pediatric emergency departments were included. The researchers concluded that “in randomized trials, POCTs had no effect on admissions (RR 0.93, 95% CI 0.61-1.42, I2 = 34%), returning for care (RR 1.00 95% CI = 0.77-1.29, I2 = 7%), or antibiotic prescribing (RR 0.97, 95% CI 0.82-1.15, I2 = 70%), but increased prescribing of antivirals (RR 2.65, 95% CI 1.95-3.60; I2 = 0%). Further testing was reduced for full blood counts (FBC) (RR 0.80, 95% CI 0.69-0.92 I2 = 0%), blood cultures (RR 0.82, 95% CI 0.68-0.99; I2 = 0%) and chest radiography (RR 0.81, 95% CI 0.68-0.96; I2 = 32%), but not urinalysis (RR 0.91, 95% CI 0.78-w1.07; I2 = 20%).” Among the non-randomized studies, fewer reported these outcomes, with some showing inconsistency with the randomized trial outcomes, such as there being fewer antibiotic prescriptions and less urinalysis testing. This demonstrated the use of POCTs for influenza and how they influence clinical treatment and decision making (Lee et al., 2019).
Kanwar et al. (2020) compared three rapid, POC molecular assays for influenza A and B detection in children: the ID Now influenza A & B assay, the Cobas influenza A/B NAAT, and Xpert Xpress Flu. Each of the three aforementioned tests are CLIA-waived influenza assays. PCR was used to compare results from each. NPS Samples from 201 children were analyzed for this study. The researchers note that “The overall sensitivities for the ID Now assay, LIAT, and Xpert assay for Flu A virus detection (93.2%, 100%, and 100%, respectively) and Flu B virus detection (97.2%, 94.4%, and 91.7%, respectively) were comparable. The specificity for Flu A and B virus detection by all methods was >97%” (Kanwar et al., 2020).
Sato et al. (2022) conducted a study comparing the results from rapid antigen detection (Quick Chaser Flu A, B), silver amplified immunochromatography (Quick Chaser Auto Flu A, B), and two separate NAATs (Xpert Xpress Flu/RSV and cobas Influenza A/B & RSV). The researchers also used a baseline RT-PCR assay as a reference for the study results. The sensitivities of the rapid antigen detection test and silver amplified immunochromatography test were 41.7% and 50.0% <6 hours after onset, but both were 100% in sensitivity at 24-48h after onset. Ultimately, the researchers concluded that the two NAATs had comparable analytical performances, whereas the rapid antigen detection and silver amplified immunochromatography tests had increased false negatives oftentimes when viral load is low in early infection.
Centers for Disease Control and Prevention (CDC)
The CDC gives two sets of guidelines concerning testing for influenza. If influenza is known to be circulating in the community, they give the algorithm displayed in the figure below (CDC, 2020b):
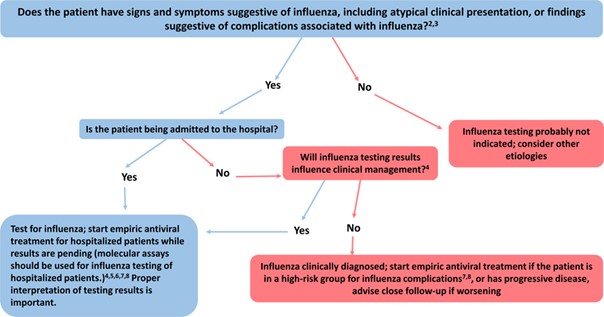
If the patient is asymptomatic for influenza, then they do not recommend testing. If the patient is symptomatic and is being admitted to the hospital, then they recommend testing; on the other hand, if a symptomatic patient is not being admitted to the hospital, they recommend testing if the results of the test will influence clinical management. Otherwise, if the test results are not going to influence the clinical management, then do not test but do administer empiric antiviral treatment for any patient in high-risk categories (CDC, 2020b). [For a list of typical signs and symptoms of influenza according to the CDC, please refer to Note 1 within the Coverage criteria section above (CDC, 2020a).]
For possible outbreaks in a closed setting or institution, the CDC issued the guideline algorithm in the figure below (CDC, 2019):
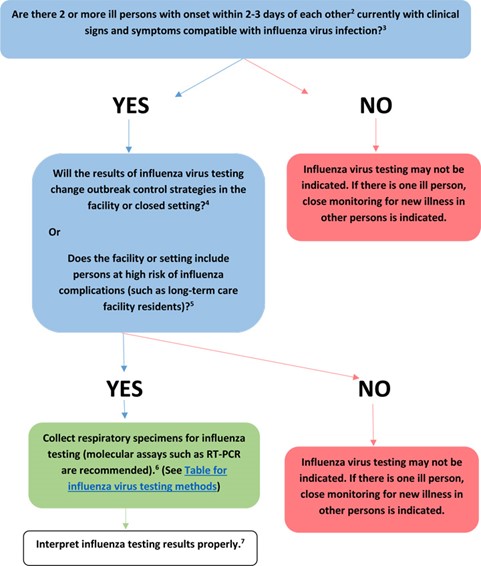
If only one person is showing signs and symptoms of influenza, then testing is not recommended but he/she should be closely monitored. If multiple people are showing signs of influenza, then RT-PCR testing is recommended if the results would change control strategies or if there are persons at high risk of complications within the facility or closed setting (CDC, 2019). [For a list of signs and symptoms and a list of high-risk populations, please see notes 1 and 2, respectively, in the Coverage criteria section above] (CDC, 2020a).
The CDC notes the usefulness of RIDT influenza testing given the rapid testing time (less than 15 minutes on the average) and that some have been cleared for point-of-care use, but they note the limited sensitivity to detect influenza as compared to the reference standards for laboratory confirmation testing, RT-PCR or viral culture. Disadvantages of RIDTs include high false negative results, especially during outbreaks, false positive results during times when influenza activity is low, and the lack of parity in RIDTs in detecting viral antigens. “Testing is not needed for all patients with signs and symptoms of influenza to make antiviral treatment decisions … Once influenza activity has been documented in the community or geographic area, a clinical diagnosis of influenza can be made for outpatients with signs and symptoms consistent with suspected influenza, especially during periods of peak influenza activity in the community” (CDC, 2017).
The CDC notes the practicality of using RIDTs to detect possible influenza outbreaks, especially in closed settings. “RIDTs can be useful to identify influenza virus infection as a cause of respiratory outbreaks in any setting, but especially in institutions (i.e., nursing homes, chronic care facilities, and hospitals), cruise ships, summer camps, schools, etc. Positive RIDT results from one or more ill persons with suspected influenza can support decisions to promptly implement infection prevention and control measures for influenza outbreaks. However, negative RIDT results do not exclude influenza virus infection as a cause of a respiratory outbreak because of the limited sensitivity of these tests. Testing respiratory specimens from several persons with suspected influenza will increase the likelihood of detecting influenza virus infection if influenza virus is the cause of the outbreak, and use of molecular assays such as RT-PCR is recommended if the cause of the outbreak is not determined and influenza is suspected. Public health authorities should be notified promptly of any suspected institutional outbreak and respiratory specimens should be collected from ill persons (whether positive or negative by RIDT) and sent to a public health laboratory for more accurate influenza testing by molecular assays and viral culture.” The CDC recommends using a molecular assay, such as RT-PCR, to test any hospitalized individual with suspected influenza rather than using an RIDT (CDC, 2017).
Infectious Diseases Society of America (IDSA)
The IDSA published an update to seasonal influenza in adults and children in 2018. The following three recommendations relating to outpatient influenza testing were published:
- “Clinicians should use rapid molecular assays (ie, nucleic acid amplification tests) over rapid influenza diagnostic tests (RIDTs) in outpatients to improve detection of influenza virus infection.”
- “Clinicians should not use viral culture for initial or primary diagnosis of influenza because results will not be available in a timely manner to inform clinical management (A-III), but viral culture can be considered to confirm negative test results from RIDTs and immunofluorescence assays, such as during an institutional outbreak, and to provide isolates for further characterization.”
- “Clinicians should not use serologic testing for diagnosis of influenza because results from a single serum specimen cannot be reliably interpreted, and collection of paired (acute/convalescent) sera 2 – 3 weeks apart are needed for serological testing” (Uyeki et al., 2018).
The 2018 IDSA guidelines for the diagnosis of infectious diseases by microbiology laboratories (Miller et al., 2018) under viral pneumonia respiratory infections, specifically including influenza, state: “Rapid antigen tests for respiratory virus detection lack sensitivity and depending upon the product, specificity. A recent meta-analysis of rapid influenza antigen tests showed a pooled sensitivity of 62.3% and a pooled specificity of 98.2%. They should be considered as screening tests only. At a minimum, a negative result should be verified by another method… Several U.S. Food and Drug Administration (FDA)-cleared NAAT platforms are currently available and vary in their approved specimen requirements and range of analytes detected” (Miller et al., 2018). Moreover, they state that the “IDSA/American Thoracic Society (ATS) practice guidelines (currently under revision) consider diagnostic testing as optional for the patient who is not hospitalized.” For children, though, they do recommend testing for viral pathogens in both outpatient and inpatient settings. In the section on general influenza virus infection, again they recommend the use of rapid testing assays, noting the higher sensitivity of the NAAT-based methods over the rapid antigen detection assays. They also state: Serologic testing is not useful for the routine diagnosis of influenza due to high rates of vaccination and/or prior exposure” (Miller et al., 2018).
American Academy of Emergency Medicine (AAEM)
The AAEM approved a clinical practice paper on influenza in the emergency department: vaccination, diagnosis, and treatment. This document gives a “Level B” recommendation that states: “Testing for influenza should only be performed if the results will change clinical management. If a RAD [rapid antigen diagnostic] testing method is utilized, the provider should be aware of the limited sensitivity and the potential for false negatives. If clinical suspicion is moderate to high and RAD test is negative, one should consider sending a confirmatory RT-PCR or proceeding with empiric treatment for suspected influenza” (Abraham et al., 2016). This guideline has since been archived on the AAEM website.
Committee on Infectious Diseases, American Academy of Pediatrics (AAP), 32nd Edition (2021-2024, Red Book)
The Committee on Infectious Diseases released joint guidelines with the American Academy of Pediatrics. These joint guidelines recommend that “influenza testing should be performed when the results are anticipated to influence clinical management (e.g., to inform the decision to initiate antiviral therapy or antibiotic agents, to pursue other diagnostic testing or to implement infection prevention and control measures)” (AAP, 2021).
Regarding types of testing, the AAP states that “The decision to test is related to the level local influenza activity, clinical suspicion for influenza, and the sensitivity and specificity of commercially available influenza tests. … These include rapid molecular assays for influenza RNA or nucleic acid detection, reverse transcriptase-polymerase chain reaction (RT-PCR) single-plex or multiplex assays, real time or other RNA-based assays, immunofluorescence assays (direct [DFA] or indirect [IFA] fluorescent antibody staining) for antigen detection, rapid influenza diagnostic tests (RIDTs) based on antigen detection, rapid cell culture (shell vial culture), and viral tissue cell culture (conventional) for virus isolation. The optimal choice of influenza test depends on the clinical setting” (AAP, 2021).
National Institute of Health (NIH)
The NIH published a webpage on influenza diagnoses. This page notes that “Diagnostics that enable healthcare professionals to quickly distinguish one flu strain from another at the point of patient care and to detect resistance to antiviral drugs would ensure that patients receive the most appropriate care” (NIH, 2017).
References
- AAP. (2021). Red Book® 2021-2024: Report of the Committee on Infectious Diseases, 32nd Edition. https://redbook.solutions.aap.org/Book.aspx?bookid=2591
- Abraham, M. K., Perkins, J., Vilke, G. M., & Coyne, C. J. (2016). Influenza in the Emergency Department: Vaccination, Diagnosis, and Treatment: Clinical Practice Paper Approved by American Academy of Emergency Medicine Clinical Guidelines Committee. J Emerg Med, 50(3), 536-542. https://doi.org/10.1016/j.jemermed.2015.10.013
- Antoniol, S., Fidouh, N., Ghazali, A., Ichou, H., Bouzid, D., Kenway, P., Choquet, C., Visseaux, B., & Casalino, E. (2018). Diagnostic performances of the Xpert(®) Flu PCR test and the OSOM(®) immunochromatographic rapid test for influenza A and B virus among adult patients in the Emergency Department. J Clin Virol, 99-100, 5-9. https://doi.org/10.1016/j.jcv.2017.12.005
- Azar, M. M., & Landry, M. L. (2018). Detection of Influenza A and B Viruses and Respiratory Syncytial Virus by Use of Clinical Laboratory Improvement Amendments of 1988 (CLIA)-Waived Point-of-Care Assays: a Paradigm Shift to Molecular Tests. J Clin Microbiol, 56(7). https://doi.org/10.1128/jcm.00367-18
- Brankston, G., Gitterman, L., Hirji, Z., Lemieux, C., & Gardam, M. (2007). Transmission of influenza A in human beings. Lancet Infect Dis, 7(4), 257-265. https://doi.org/10.1016/s1473-3099(07)70029-4
- Call, S. A., Vollenweider, M. A., Hornung, C. A., Simel, D. L., & McKinney, W. P. (2005). Does this patient have influenza? Jama, 293(8), 987-997. https://doi.org/10.1001/jama.293.8.987
- CDC. (2017). Rapid Influenza Diagnostic Tests. https://www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm
- CDC. (2019, 03/04/2019). Influenza virus testing in investigational outbreaks in institutional or other closed settings. Centers for Disease Control and Prevention. https://www.cdc.gov/flu/professionals/diagnosis/guide-virus-diagnostic-tests.htm
- CDC. (2020a, 08/31/2020). Algorithm to Assist in Medical Office Telephone Evaluation of Patients with Possible Influenza. Centers for Disease Control and Prevention. Retrieved 07/08/2022 from https://www.cdc.gov/flu/professionals/antivirals/office-evaluation.htm
- CDC. (2020b, September 1). Guide for considering influenza testing when influenza viruses are circulating in the community. Centers for Disease Control and Prevention. https://www.cdc.gov/flu/professionals/diagnosis/consider-influenza-testing.htm
- Chartrand, C., Leeflang, M. M., Minion, J., Brewer, T., & Pai, M. (2012). Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med, 156(7), 500-511. https://doi.org/10.7326/0003-4819-156-7-201204030-00403
- CMS. (2018, 01/04/2018). TESTS GRANTED WAIVED STATUS UNDER CLIA. Centers for Medicare & Medicaid Services. Retrieved 07/24/2018 from https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Downloads/waivetbl.pdf
- Cooper, N. J., Sutton, A. J., Abrams, K. R., Wailoo, A., Turner, D., & Nicholson, K. G. (2003). Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. Bmj, 326(7401), 1235. https://doi.org/10.1136/bmj.326.7401.1235
- Cowling, B. J., Chan, K. H., Fang, V. J., Lau, L. L., So, H. C., Fung, R. O., Ma, E. S., Kwong, A. S., Chan, C. W., Tsui, W. W., Ngai, H. Y., Chu, D. W., Lee, P. W., Chiu, M. C., Leung, G. M., & Peiris, J. S. (2010). Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med, 362(23), 2175-2184. https://doi.org/10.1056/NEJMoa0911530
- Cox, N. J., & Subbarao, K. (1999). Influenza. Lancet, 354(9186), 1277-1282. https://doi.org/10.1016/s0140-6736(99)01241-6
- Dobson, J., Whitley, R. J., Pocock, S., & Monto, A. S. (2015). Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet, 385(9979), 1729-1737. https://doi.org/10.1016/s0140-6736(14)62449-1
- Dolin, R. (1976). Influenza: current concepts. Am Fam Physician, 14(3), 72-77.
- Dolin, R. (2022a, 04/01/2022). Seasonal influenza in adults: Clinical manifestations and diagnosis. https://www.uptodate.com/contents/seasonal-influenza-in-adults-clinical-manifestations-and-diagnosis
- Dolin, R. (2022b, 04/01/2022). Seasonal influenza in adults: Transmission, clinical manifestations, and complications. https://www.uptodate.com/contents/seasonal-influenza-in-adults-transmission-clinical-manifestations-and-complications
- Harper, S. A., Bradley, J. S., Englund, J. A., File, T. M., Gravenstein, S., Hayden, F. G., McGeer, A. J., Neuzil, K. M., Pavia, A. T., Tapper, M. L., Uyeki, T. M., & Zimmerman, R. K. (2009). Seasonal influenza in adults and children--diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis, 48(8), 1003-1032. https://doi.org/10.1086/598513
- Hayden, F. G., Osterhaus, A. D., Treanor, J. J., Fleming, D. M., Aoki, F. Y., Nicholson, K. G., Bohnen, A. M., Hirst, H. M., Keene, O., & Wightman, K. (1997). Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med, 337(13), 874-880. https://doi.org/10.1056/nejm199709253371302
- Hazelton, B., Gray, T., Ho, J., Ratnamohan, V. M., Dwyer, D. E., & Kok, J. (2015). Detection of influenza A and B with the Alere i Influenza A & B: a novel isothermal nucleic acid amplification assay. Influenza Other Respir Viruses, 9(3), 151-154. https://doi.org/10.1111/irv.12303
- Heneghan, C. J., Onakpoya, I., Thompson, M., Spencer, E. A., Jones, M., & Jefferson, T. (2014). Zanamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. Bmj, 348, g2547. https://doi.org/10.1136/bmj.g2547
- Hurt, A. C., Alexander, R., Hibbert, J., Deed, N., & Barr, I. G. (2007). Performance of six influenza rapid tests in detecting human influenza in clinical specimens. J Clin Virol, 39(2), 132-135. https://doi.org/10.1016/j.jcv.2007.03.002
- Ikenaga, M., Kosowska-Shick, K., Gotoh, K., Hidaka, H., Koga, H., Masunaga, K., Nagai, K., Tsumura, N., Appelbaum, P. C., & Matsuishi, T. (2008). Genotypes of macrolide-resistant pneumococci from children in southwestern Japan: raised incidence of strains that have both erm(B) and mef(A) with serotype 6B clones. Diagn Microbiol Infect Dis, 62(1), 16-22. https://doi.org/10.1016/j.diagmicrobio.2007.10.013
- Jefferson, T., Jones, M., Doshi, P., Spencer, E. A., Onakpoya, I., & Heneghan, C. J. (2014). Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. Bmj, 348, g2545. https://doi.org/10.1136/bmj.g2545
- Kanwar, N., Michael, J., Doran, K., Montgomery, E., & Selvarangan, R. (2020). Comparison of the ID Now Influenza A & B 2, Cobas Influenza A/B, and Xpert Xpress Flu Point-of-Care Nucleic Acid Amplification Tests for Influenza A/B Virus Detection in Children. J Clin Microbiol, 58(3). https://doi.org/10.1128/jcm.01611-19
- Kilbourne, E. D., & Loge, J. P. (1950). Influenza A prime: a clinical study of an epidemic caused by a new strain of virus. Ann Intern Med, 33(2), 371-379.
- Kux, L. (2017). Microbiology Devices; Reclassification of Influenza Virus Antigen Detection Test Systems Intended for Use Directly With Clinical Specimens. (FDA-2014-N-0440). Washington, D.C.: Federal Register Retrieved from https://www.gpo.gov/fdsys/pkg/FR-2017-01-12/pdf/2017-00199.pdf
- Lee, J. J., Verbakel, J. Y., Goyder, C. R., Ananthakumar, T., Tan, P. S., Turner, P. J., Hayward, G., & Van den Bruel, A. (2019). The Clinical Utility of Point-of-Care Tests for Influenza in Ambulatory Care: A Systematic Review and Meta-analysis. Clin Infect Dis, 69(1), 24-33. https://doi.org/10.1093/cid/ciy837
- Ling, L., Kaplan, S. E., Lopez, J. C., Stiles, J., Lu, X., & Tang, Y. W. (2018). Parallel Validation of Three Molecular Devices for Simultaneous Detection and Identification of Influenza A and B and Respiratory Syncytial Viruses. J Clin Microbiol, 56(3). https://doi.org/10.1128/jcm.01691-17
- Loeb, M., Singh, P. K., Fox, J., Russell, M. L., Pabbaraju, K., Zarra, D., Wong, S., Neupane, B., Singh, P., Webby, R., & Fonseca, K. (2012). Longitudinal study of influenza molecular viral shedding in Hutterite communities. J Infect Dis, 206(7), 1078-1084. https://doi.org/10.1093/infdis/jis450
- Lopez Roa, P., Catalan, P., Giannella, M., Garcia de Viedma, D., Sandonis, V., & Bouza, E. (2011). Comparison of real-time RT-PCR, shell vial culture, and conventional cell culture for the detection of the pandemic influenza A (H1N1) in hospitalized patients. Diagn Microbiol Infect Dis, 69(4), 428-431. https://doi.org/10.1016/j.diagmicrobio.2010.11.007
- Melchers, W. J. G., Kuijpers, J., Sickler, J. J., & Rahamat-Langendoen, J. (2017). Lab-in-a-tube: Real-time molecular point-of-care diagnostics for influenza A and B using the cobas(R) Liat(R) system. J Med Virol, 89(8), 1382-1386. https://doi.org/10.1002/jmv.24796
- Miller, J. M., Binnicker, M. J., Campbell, S., Carroll, K. C., Chapin, K. C., Gilligan, P. H., Gonzalez, M. D., Jerris, R. C., Kehl, S. C., Patel, R., Pritt, B. S., Richter, S. S., Robinson-Dunn, B., Schwartzman, J. D., Snyder, J. W., Telford, I. I. I. S., Theel, E. S., Thomson, J. R. B., Weinstein, M. P., & Yao, J. D. (2018). A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiologya. Clinical Infectious Diseases, ciy381-ciy381. https://doi.org/10.1093/cid/ciy381
- Moesker, F. M., van Kampen, J. J. A., Aron, G., Schutten, M., van de Vijver, D., Koopmans, M. P. G., Osterhaus, A., & Fraaij, P. L. A. (2016). Diagnostic performance of influenza viruses and RSV rapid antigen detection tests in children in tertiary care. J Clin Virol, 79, 12-17. https://doi.org/10.1016/j.jcv.2016.03.022
- Mubareka, S., Lowen, A. C., Steel, J., Coates, A. L., Garcia-Sastre, A., & Palese, P. (2009). Transmission of influenza virus via aerosols and fomites in the guinea pig model. J Infect Dis, 199(6), 858-865.
- Nicholson, K. G. (1992). Clinical features of influenza. Semin Respir Infect, 7(1), 26-37.
- Nicholson, K. G., Aoki, F. Y., Osterhaus, A. D., Trottier, S., Carewicz, O., Mercier, C. H., Rode, A., Kinnersley, N., & Ward, P. (2000). Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet, 355(9218), 1845-1850.
- NIH. (2017, April 10). Influenza Diagnosis. https://www.niaid.nih.gov/diseases-conditions/influenza-diagnosis
- Ryu, S. W., Lee, J. H., Kim, J., Jang, M. A., Nam, J. H., Byoun, M. S., & Lim, C. S. (2016). Comparison of two new generation influenza rapid diagnostic tests with instrument-based digital readout systems for influenza virus detection. Br J Biomed Sci, 73(3), 115-120. https://doi.org/10.1080/09674845.2016.1189026
- Ryu, S. W., Suh, I. B., Ryu, S. M., Shin, K. S., Kim, H. S., Kim, J., Uh, Y., Yoon, K. J., & Lee, J. H. (2018). Comparison of three rapid influenza diagnostic tests with digital readout systems and one conventional rapid influenza diagnostic test. J Clin Lab Anal, 32(2). https://doi.org/10.1002/jcla.22234
- Sato, Y., Nirasawa, S., Saeki, M., Yakuwa, Y., Ono, M., Kobayashi, R., Nakafuri, H., Murai, R., Fujiya, Y., Kuronuma, K., & Takahashi, S. (2022). Comparative study of rapid antigen testing and two nucleic acid amplification tests for influenza virus detection. J Infect Chemother, 28(7), 1033-1036. https://doi.org/10.1016/j.jiac.2022.04.009
- Sintchenko, V., Gilbert, G. L., Coiera, E., & Dwyer, D. (2002). Treat or test first? Decision analysis of empirical antiviral treatment of influenza virus infection versus treatment based on rapid test results. J Clin Virol, 25(1), 15-21.
- Uyeki, T. M., Bernstein, H. H., Bradley, J. S., Englund, J. A., File, T. M., Jr., Fry, A. M., Gravenstein, S., Hayden, F. G., Harper, S. A., Hirshon, J. M., Ison, M. G., Johnston, B. L., Knight, S. L., McGeer, A., Riley, L. E., Wolfe, C. R., Alexander, P. E., & Pavia, A. T. (2018). Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clinical Infectious Diseases, 68(6), e1-e47. https://doi.org/10.1093/cid/ciy866
- Yoon, J., Yun, S. G., Nam, J., Choi, S. H., & Lim, C. S. (2017). The use of saliva specimens for detection of influenza A and B viruses by rapid influenza diagnostic tests. J Virol Methods, 243, 15-19. https://doi.org/10.1016/j.jviromet.2017.01.013
- Young, S., Illescas, P., Nicasio, J., & Sickler, J. J. (2017). Diagnostic accuracy of the real-time PCR cobas((R)) Liat((R)) Influenza A/B assay and the Alere i Influenza A&B NEAR isothermal nucleic acid amplification assay for the detection of influenza using adult nasopharyngeal specimens. J Clin Virol, 94, 86-90. https://doi.org/10.1016/j.jcv.2017.07.012
- Zachary, K. C. (2022, 06/29/2022). Seasonal influenza in nonpregnant adults: Treatment. https://www.uptodate.com/contents/seasonal-influenza-in-nonpregnant-adults-treatment
Coding Section
|
CPT |
|
Code Description |
|
|
86710 |
Antibody; influenza virus |
|
|
87254 |
Virus isolation; centrifuge enhanced (shell vial) technique, includes identification with immunofluorescence stain, each virus |
|
|
87275 |
Infectious agent antigen detection by immunofluorescent technique; influenza B virus |
|
|
87276 |
Infectious agent antigen detection by immunofluorescent technique; influenza A virus |
|
|
87400 |
Infectious agent antigen detection by immunoassay technique, (e.g., enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], immunochemiluminometric assay [IMCA]) qualitative or semiquantitative, multiple-step method; Influenza, A or B, each |
|
|
87501 |
Infectious agent detection by nucleic acid (DNA or RNA); influenza virus, includes reverse transcription, when performed, and amplified probe technique, each type or subtype |
|
|
87502 |
Infectious agent detection by nucleic acid (DNA or RNA); influenza virus, for multiple types or sub-types, includes multiplex reverse transcription, when performed, and multiplex amplified probe technique, first 2 types or sub-types |
|
|
87503 |
Infectious agent detection by nucleic acid (DNA or RNA); influenza virus, for multiple types or sub-types, includes multiplex reverse transcription, when performed, and multiplex amplified probe technique, each additional influenza virus type or sub-type beyond 2 (List separately in addition to code for primary procedure) |
|
|
87631 |
Infectious agent detection by nucleic acid (DNA or RNA); respiratory virus (e.g., adenovirus, influenza virus, coronavirus, metapneumovirus, parainfluenza virus, respiratory syncytial virus, rhinovirus), includes multiplex reverse transcription, when performed, and multiplex amplified probe technique, multiple types or subtypes, 3 – 5 targets |
|
|
87804 |
Infectious agent antigen detection by immunoassay with direct optical observation; Influenza |
|
ICD-10 |
|
|
|
|
R05.1 |
Acute cough |
|
|
R05.2 |
Subacute cough |
|
|
R05.3 |
Chronic cough |
|
|
R05.4 |
Cough syncope |
|
|
R05.8 |
Other specified cough |
|
|
R05.9 |
Cough, unspecified |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, Blue Cross Blue Shield Association technology assessment program (TEC) and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2016 Forward
| 10/21/2022 | Annual review, no change to policy intent. Policy verbiage reformatted for clarity. Updating table of terminology, rationale, references. |
|
10/08/2021 |
Annual review, no change to policy intent. Updating background, rationale, references and ICD 10 coding. |
|
10/01/2020 |
Annual review, no change to policy intent. Updating description, coding, rationale and references. |
|
10/10/2019 |
Annual review, no change to policy intent. |
|
11/15/2018 |
Annual review, policy being revised to encompass more than Rapid Flu testing. Updating title, policy verbiage and coding. |
|
10/30/2017 |
Updated Coding section. No other changes. |
|
09/28/2017 |
Updated coding section with 2018 coding. No other changes. |
|
06/19/2017 |
Updating coding section. No other changes made. |
|
04/26/2017 |
Updated category to Laboratory. No other changes. |
|
03/07/2017 |
Updated coding section. |
|
01/03/2017 |
Annual review, no change to policy intent. |
|
01/11/2016 |
NEW POLICY |