Diagnostic Testing of Most Common Sexually Transmitted Infections - CAM 209
Description
Sexually transmitted infections (STIs), often referred to as sexually transmitted diseases or STDs, include a variety of pathogenic bacteria, virus, and other microorganisms that are spread through sexual contact and can cause a multitude of complications if left untreated. Chlamydia and gonorrhea, caused by Chlamydia trachomatis and Neisseria gonorrhoeae, respectively, have high rates of occurrence in the United States and can cause pelvic inflammatory disease (PID), infertility and pregnancy complications. The causative agent of syphilis is Treponema pallidum; if left untreated, syphilis can lead to serious cardiac and neurological conditions (Ghanem & Tuddenham, 2021b). Trichmoniasis is a common genitourinary infection caused by Trichomonas vaginalis. This infection is the most common cause of vaginal complaints, but other areas such as the prostate and bladder may be affected (Sobel, 2020). Human papillomavirus (HPV) is a double-stranded DNA virus that can be sexually transmitted and is associated with cervical cancer, vulvar/vaginal cancer, anal cancer, oropharyngeal cancer, penile cancer, and both genital and nongenital warts. “Globally, anogenital HPV is the most common sexually transmitted infection” with an estimated 80% of sexually active adults exposed to it at least once in their lifetime (Palefsky, 2019). Herpes simplex virus (HSV) is a common STI where many individuals are asymptomatic. HSV infection has been linked to an increased risk of other infections, including HIV, and in rare cases, can also result in HSV meningitis or proctitis (Albrecht, 2018). In general, risk factors for STIs can include both behavioral elements, such as multiple sex partners, working in a sex trade, and inconsistent use of condoms when in non-monogamous relationships as well as demographic risks, including men who have sex with men (MSM), prior STI diagnosis, admission to correctional facilities, and lower socioeconomic status (Ghanem & Tuddenham, 2021a).
This policy is limited to testing for C. trachomatis, N. gonorrhoeae, T. pallidum, T. vaginalis, HSV, and HPV. The following conditions and/or tests are discussed in the corresponding policies:
- HIV Genotyping and Phenotyping; CAM 302
- Hepatitis C: CAM 127
- Preventive Screening: CAM 089
- Pediatric Preventive Screening: CAM 109
- Prenatal Screening: CAM 119
- Cervical Cancer Screening: CAM 20409
- Pathogen Panel Testing: CAM 181
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
- Antibody testing for syphilis infection is considered MEDICALLY NECSSESSARY in the following situations:
- For any asymptomatic person in a high-risk category (see Notes 1 & 2), once a year assessment using either a “standard” or “reverse” algorithm that includes initial and confirmatory tests for any initial positive test, such as:
- Treponemal Ig test.
- Nontreponemal Ig test.
- For diagnosis of any person presenting with signs and/or symptoms of a syphilis infection (see Note 3).
- Once every three months for HIV-positive men or MSM.
- When a nontreponemal test is used as a test of cure (TOC) for a positive syphilis infection.
- For any asymptomatic person in a high-risk category (see Notes 1 & 2), once a year assessment using either a “standard” or “reverse” algorithm that includes initial and confirmatory tests for any initial positive test, such as:
- For asymptomatic individuals NOT belonging to a high-risk category (see Notes 1 & 2), antibody screening for syphilis is considered MEDICALLY NECSSESSARY only in the following situations:
- As part of newborn screening
- As part of follow-up in a victim of sexual assault
- For sexually active individuals less than 18 years of age (annually)
- Polymerase chain reaction (PCR) testing and nucleic acid amplification testing (NAAT) for syphilis is considered NOT MEDICALLY NECSSESSARY.
- NAAT for chlamydia is considered MEDICALLY NECSSESSARY in the following situations:
- Once a year assessment for any asymptomatic person in a high-risk category (see Notes 1 & 4)
- For diagnosis of any person presenting with signs and/or symptoms of a chlamydial infection (see Note 5)
- For the diagnosis of any person with suspected lymphogranuloma venereum (LGV)
- At least three months after initial chlamydial diagnosis as a TOC
- For asymptomatic individuals NOT belonging to a high-risk category (see Notes 1 & 4), screening for chlamydia is considered MEDICALLY NECSSESSARY only in the following situations:
- As part of newborn screening
- As part of follow-up in a victim of sexual assault
- For sexually active individuals less than 18 years of age (annually)
- Serology testing for chlamydia or LGV is considered NOT MEDICALLY NECSSESSARY.
- NAAT for gonorrhea is considered MEDICALLY NECSSESSARY in the following situations:
- Once a year assessment for any asymptomatic person in a high-risk category (see Notes 1 & 4)
- For diagnosis of any person presenting with signs and/or symptoms of a gonorrheal infection (see Note 6)
- As a TOC for treatment
- For an individual that does not respond to initial treatment, culture testing for N. gonorrhoeae to determine antimicrobial susceptibility is considered MEDICALLY NECSSESSARY.
- For asymptomatic individuals NOT belonging to a high-risk category (see Notes 1 & 4), screening for gonorrhea is considered MEDICALLY NECSSESSARY only in the following situations:
- As part of newborn screening
- As part of follow-up in a victim of sexual assault
- For sexually active individuals less than 18 years of age (annually)
- When an individual meets the conditions described above for both chlamydia and gonorrhea, multitarget PCR testing (targets limited to C. trachomatis and N. gonorrhoeae) is considered MEDICALLY NECSSESSARY.
- For individuals with active genital ulcers or mucocutaneous lesions, nucleic acid amplification testing (NAAT) for herpes simplex virus-1 (HSV-1) or herpes simplex virus-2 (HSV-2) is considered MEDICALLY NECSSESSARY.
- Immunoassay testing for HSV-1 and and/or herpes simplex (non-specific type test) is considered NOT MEDICALLY NECSSESSARY.
- Type-specific serologic testing for HSV-2 using a glycoprotein G2 (gG2) test is considered MEDICALLY NECSSESSARY in the following situations:
- Recurrent or atypical genital symptoms or lesions in individuals with a negative herpes simplex virus PCR or culture result
- For the clinical diagnosis of genital herpes in individuals with a negative PCR or culture result or without laboratory confirmation
- When an individual’s partner has genital herpes
- In asymptomatic individuals, screening for HSV-1 or HSV-2 is considered NOT MEDICALLY NECSSESSARY.
- In the diagnosis and/or assessment of cancer or cancer therapy (immunohistochemistry testing for p16 or NAAT testing for high-risk human papillomavirus [HR-HPV]), testing for HR-HPV is considered MEDICALLY NECSSESSARY.
- Testing for HPV is considered NOT MEDICALLY NECSSESSARY in the following situations:
- To screen for oncogenic high-risk types, such as HPV-16 and HPV-18, as part of a general sexually transmitted disease (STD) or sexually transmitted infection (STI) screening process or panel for asymptomatic individuals
- As part of the diagnosis of anogenital warts
- To screen for low-risk types of HPV
- In the general population, either as a part of a panel of tests or as an individual NAAT to determine HPV status
- NAATs or PCR-based testing for T. vaginalis is considered MEDICALLY NECSSESSARY in the following situations:
- Symptomatic individuals (see Note 7)
- Asymptomatic individuals belonging to a high-risk group:
- Concurrent STI or history of STIs
- Individuals in high prevalence settings, such as STI clinics
- Individuals who exchange sex for payment
- Rapid identification of Trichomonas by enzyme immunoassay is considered NOT MEDICALLY NECSSESSARY.
- Prior to beginning a preexposure prophylaxis (PrEP) regimen, the following screens/tests is considered MEDICALLY NECSSESSARY:
- Serum creatinine and estimated creatinine clearance to determine baseline renal function
- Antibody screening to confirm a baseline negative antibody result for HIV
- Hepatitis B (HBV) and/or Hepatitis C screening to identify positive individuals
- Pregnancy testing
- While an individual is undergoing a preexposure prophylaxis (PrEP) regimen for HIV prevention, the following screens/tests is considered MEDICALLY NECSSESSARY:
- A blood test once every three months to confirm a negative antibody result for HIV
- Serum creatinine and estimated creatinine clearance three months after beginning PrEP and up to one time every six months thereafter to assess renal function
- NAAT screening, based on anatomic site of exposure, for gonorrhea and chlamydia:
- Once every three months for MSM and for individuals with child-bearing potential
- Nine months after PrEP is initiated and once every six months thereafter for sexually active individuals
- Blood test to screen for syphilis once every three months in MSM and individuals with child-bearing potential
- Once every three months for MSM and for individuals with child-bearing potential
- Nine months after PrEP is initiated and once every six months thereafter for sexually active individuals
- Pregnancy testing once every three months
The following does not meet coverage criteria due to a lack of available published scientific literature confirming that the test(s) is/are required and beneficial for the diagnosis and treatment of a patient’s illness.
- Using nucleic acid testing to quantify the following microorganisms is considered NOT MEDICALLY NECSSESSARY:
- Chlamydia trachomatis
- Neisseria gonorrhoeae
- Herpes Simplex Virus-1
- Herpes Simplex Virus-2
- Human Papillomavirus
- Treponema pallidum
NOTES:
Note 1: For sexually active children and adolescents under the age of 18, risk factors for chlamydia, gonorrhea, and/or syphilis infection as defined by the CDC include: initiating sex early in adolescence; living in detention facilities; receiving services at STD clinics; being involved in commercial sex exploitation or exchanging sex for drugs, money, food, or housing; having multiple sex partners, having sequential sex partnerships of limited duration or concurrent partnerships; failing to use barrier protection consistently and correctly; having lower socioeconomic status, and facing numerous obstacles to accessing healthcare. At-risk individuals also include: males who have sex with males (YMSM); transgender youths; youths with disabilities, substance abuse, or mental health disorders (CDC, 2022b).
Note 2: High-risk for Syphilis (Cantor et al., 2016; CDC, 2021g):
- Sexually active men who have sex with men (MSM)
- Sexually active HIV-positive status
- Having a sexual partner recently diagnosed with an STI
- Exchanging sex for money or drugs
- Individuals in adult correctional facilities
- During pregnancy when the following risk factors are present:
- Sexually active HIV-positive status
- Sexually active with multiple partners
- Sexually active in conjunction with drug use or transactional sex
- Late entry to prenatal care (i.e., first visit during the second trimester or later) or no prenatal care
- Methamphetamine or heroin use
- Incarceration of the woman or her partner
- Unstable housing or homelessness
Note 3: Signs and Symptoms of a Syphilis Infection (CDC, 2021g)
- Chancre
- Skin rash and/or mucous membrane lesions in mouth, vagina, anus, hands, and feet
- Condyloma lata
- Secondary symptomology can include fever, fatigue, sore throat, swollen lymph nodes, weight loss, muscle aches, headache, and hair loss
Note 4: High-risk for Chlamydia and/or Gonorrhea (CDC, 2021a, 2021d, 2021f; LeFevre, 2014):
- Sexually active men who have sex with men (MSM)
- Sexually active HIV-positive status
- Sexually active women under the age of 25
- Women age 25 or over who have multiple sexual partners
- Having a sexual partner recently diagnosed with an STI
- Previous or concurrent STI
- Exchanging sex for money or drugs
Note 5: Signs and Symptoms of a Chlamydia Infection (CDC, 2021a, 2021f):
- Genital symptoms, including “discharge, burning during urination, unusual sores, or rash”
- Pelvic Inflammatory Disease, including “symptoms of abdominal and/or pelvic pain, along with signs of cervical motion tenderness, and uterine or adnexal tenderness on examination”
- Urethritis
- Pyuria
- Dysuria
- Increase in frequency in urination
- Epididymitis (with or without symptomatic urethritis) in men
- Proctitis
- Sexually acquired chlamydial conjunctivitis
Note 6: Signs and Symptoms of Gonorrhea (CDC, 2021d):
- Dysuria
- Urethral infection
- Urethral or vaginal discharge
- Epididymitis (Testicular or scrotal pain)
- Rectal infection symptoms include anal itching, discharge, rectal bleeding, and painful bowel movements
NOTE 7: Signs and Symptoms of Trichomoniasis (CDC, 2021h, 2021i):
- Vaginal or penile discharge
- Itching, burning sensation, or soreness of the genitalia
- Discomfort or burning sensation during/after urination and/or ejaculation
- Urethritis
- Epididymitis
- Prostatitis
Terms of Terminology
|
Term |
Definition |
|
AAP |
American Academy of Pediatrics |
|
AIDs |
Acquired immune deficiency syndrome |
|
AIN |
Anal intraepithelial neoplasia |
|
ASCUS |
Atypical squamous cells of undetermined significance |
|
BASHH |
British Association for Sexual Health and HIV |
|
BD |
Becton Dickinson |
|
CDC |
Centers for Disease Control and Prevention |
|
CI |
Confidence interval |
|
CIA |
Chemiluminescence immunoassay |
|
CIN2+ |
Cervical intraepithelial neoplasia grade 2+ |
|
CIN3 |
Cervical intraepithelial neoplasia grade 3 |
|
CLIA |
Chemiluminescent assay |
|
CLIA ’88 |
Clinical Laboratory Improvement Amendments of 1988 |
|
CMIA |
Chemiluminescence immunoassay |
|
CMS |
Centers for Medicare & Medicaid |
|
CNS |
Central nervous system |
|
CPS |
Canadian Paediatric Society |
|
CSF |
Cerebrospinal fluid |
|
CT |
Chlamydia trachomatis |
|
DFE |
Darkfield examination |
|
DNA |
Deoxyribonucleic acid |
|
DRE |
Digital rectal examination |
|
E7-MPG |
E7 multiplex genotyping |
|
EBV |
Epstein Barr virus |
|
ED |
Emergency department |
|
EIA |
Enzyme immunoassay |
|
ELISA |
Enzyme-linked immunosorbent assay |
|
FDA |
Food and Drug Administration |
|
FEMS |
Federation of European Microbiological Societies |
|
FIA |
Fluorescence immunoassay |
|
FNA |
Fine needle aspiration |
|
FTA |
Fluorescent treponemal antibody |
|
GC |
Gonococcal |
|
gG2 |
Glycoprotein G2 |
|
GP5+/6+ |
General primer 5+/6+ |
|
HBV |
Hepatitis B |
|
HC2 |
Hybrid capture 2 |
|
hCG |
Human chorionic gonadotropin |
|
HIV |
Human immunodeficiency virus |
|
HIV-1 |
Human immunodeficiency virus-1 |
|
HPV |
Human papillomavirus |
|
HPV-16 |
Human papillomavirus type 16 |
|
HPV-18 |
Human papillomavirus type 18 |
|
HR-HPV |
High risk or oncogenic HPV testing |
|
HSIL |
High-grade squamous intraepithelial lesion |
|
HSV |
Herpes simplex virus |
|
HSV-1 |
Herpes simplex virus-1 |
|
HSV-2 |
Herpes simplex virus-2 |
|
IgG |
Immunoglobulin G |
|
IgM |
Immunoglobulin M |
|
IHC |
Immunohistochemistry |
|
IMCA |
Immunochemiluminometric assay |
|
ISH |
In situ hybridization |
|
ISVVD |
The International Society for the Study of Vulvovaginal Disease |
|
IUSTI |
International Union Against Sexually Transmitted Infections |
|
JAMA |
Journal of the American Medical Association |
|
LDTs |
Laboratory-Developed Tests |
|
LGSIL |
Low grade squamous intraepithelial lesion on cytologic smear of anus |
|
LGV |
Lymphogranuloma venereum |
|
LSIL |
Low-grade squamous intraepithelial lesions |
|
MHA-TP |
Microhemagglutination Assay for Treponema pallidum antibodies |
|
MLST |
Multilocus sequence typing |
|
mRNA |
Messenger RNA |
|
MSM |
Men having sex with men |
|
MTC |
Male Training Center for Family Planning & Reproductive Health |
|
NA |
Not applicable |
|
NAAT |
Nucleic acid amplification testing |
|
NCCN |
National Comprehensive Cancer Network |
|
NICE |
National Institute for Health and Care Excellence |
|
NOS |
Not otherwise specified |
|
NTT |
Nontreponemal test |
|
ORPH-1 |
Oropharynx-1 |
|
OS |
Overall survival |
|
PCR |
Polymerase chain reaction |
|
PID |
Pelvic inflammatory disease |
|
POC |
Point-of-care |
|
POCTs |
Point-of-care tests |
|
PrEP |
Preexposure prophylaxis |
|
PWID |
People who inject drugs |
|
RFLP |
Restriction fragment length polymorphism |
|
RNA |
Ribonucleic acid |
|
RPR |
Rapid plasma reagin test |
|
SDA |
Strand displacement amplification |
|
STDs |
Sexually transmitted diseases |
|
STIs |
Sexually transmitted infections |
|
TMA |
Transcription-mediated amplification |
|
TPHA |
Treponema pallidum hemagglutination |
|
TP-IgA |
Treponema pallidum IgA antibodies |
|
TPPA |
Treponema pallidum particle agglutination |
|
TP-PA |
T. pallidum passive particle agglutination |
|
TOC |
Test of cure |
|
TT |
Treponemal test |
|
USPSTF |
United States Preventive Services Task Force |
|
VDRL |
Venereal disease research laboratory |
|
VIN |
Vulvar intraepithelial neoplasia |
Rationale
Chlamydia
Chlamydia, caused by the bacterium Chlamydia trachomatis, is usually an asymptomatic sexually transmitted infection that can be passed to a newborn from an infected mother, potentially resulting in conjunctivitis and/or pneumonia. Symptomatic infections can include cervicitis, pelvic inflammatory disease (PID), and Fitzhugh-Curtis syndrome in women as well as epididymitis, prostatitis, and reactive arthritis triad in men. Both men and women can have proctitis, urethritis, conjunctivitis, pharyngitis, and genital lymphogranuloma venereum as a result of a chlamydial infection. Nucleic acid amplification testing (NAAT) for chlamydia is the gold standard due to high specificity and sensitivity instead of using culture testing, microscopy, or antigen detection (Hsu, 2022). In the U.S. alone, in 2018, over 1.7 million cases of chlamydia were reported to the CDC, but the CDC estimates that 2.86 million chlamydial infections occur annually (CDC, 2021a). This under-reporting is due to individuals who are asymptomatic and, therefore, do not seek treatment. Highest prevalence occurs among men who have sex with men (MSM) and young people. “It is estimated that 1 in 20 sexually active young women aged 14 – 24 years has chlamydia” (CDC, 2021a).
Gonorrhea
Gonorrhea is a sexually transmitted infection caused by the bacterium Neisseria gonorrhoeae. A gonorrheal infection can cause many of the same complications as chlamydia, including PID, cervicitis, and Fitzhugh-Curtis syndrome in women and epididymitis in men. Urethritis, pharyngitis, and proctitis can also occur; in fact, “N. gonorrhoeae can be isolated from the urethra in up to 90 percent of women with gonococcal cervicitis” (Ghanem, 2022). Like chlamydia, if left untreated, gonorrhea can be spread from mother to newborn, resulting in conjunctivitis. NAAT is the best method to diagnose gonorrhea, but culture testing is still used to determine antimicrobial susceptibility due to an increase in antibiotic resistance (Unemo, 2020). In 2016, the CDC reported an 18.5% increase since 2015 in the number of cases of gonorrhea reported in the United States (CDC, 2017). The CDC also reported 583,405 new cases of gonorrhea in the United States in 2018 (CDC, 2021d).
Syphilis
Syphilis is caused by the bacterium Treponema pallidum, and it progresses, if left untreated, through various stages — primary, secondary, early-latent, late-latent, and late-stage syphilis — until infecting the central nervous system. “Syphilis infection is associated with HIV infection and increases the risk for acquiring or spreading HIV” (Cantor et al., 2016). Worldwide, the median rates of infection in males and females were 17.7 cases per 100,000 and 17.2 cases per 100,000, respectively, according to the World Health Organization. The U.S. has reported an increase in the rate of syphilis between 2000 and 2016, and approximately 90% of the new cases of primary and secondary syphilis during this period occurred in men with 81% occurring in men who have sex with men (MSM). Of concern, there has also been an increased number of cases of syphilis in women. In 2018, 1306 cases of congenital syphilis were reported. This included 78 syphilitic stillbirths and 16 infant deaths. (Hicks & Clement, 2021a).
Similar to other STIs, syphilis is often asymptomatic. For symptomatic syphilis, the signs and symptoms can vary, depending on the stage of disease. Primary syphilis can have a characteristic chancre, a skin lesion that is usually painless and often heals even in the absence of treatment. Secondary syphilis occurs weeks to months later and can be manifested by typical immunologic responses, such as fever, lethargy, and so on; adenopathy; rash; alopecia; hepatitis; gastrointestinal abnormalities; and even early symptoms of neurological infection, if left untreated. Later stages of syphilis can include cardiovascular abnormalities and progression of neurological syphilitic infection. Asymptomatic, latent syphilis can also occur; moreover, “pregnant women with latent syphilis can transmit T. pallidum to their fetus for up to four years after acquisition” (Hicks & Clement, 2021a).
The standard protocol for diagnosing a syphilis infection is to use a two-tiered serological testing algorithm of treponemal testing and nontreponemal testing. Treponemal testing is typically more complex than the latter, and they both rely upon the detection of specific treponemal antigens using enzyme immunoassay (EIA), particle agglutination assay, fluorescence, or chemiluminescence immunoassay (CIA). Nontreponemal testing methods, including the rapid plasma reagin test (RPR) and the venereal disease research laboratory (VDRL) test, “are based upon the reactivity of serum from infected patients to a cardiolipin-cholesterol-lecithin antigen” (Hicks & Clement, 2021b). Rapid serological testing using darkfield microscopy is not as universally used due to complexity and cost. NAAT has not been FDA-approved at this time and is not typically performed for genital syphilis. “There is no internationally approved PCR for T. pallidum and accordingly, it is crucial to select a strictly validated method and always use it with appropriate quality controls” (Janier et al., 2014).
Herpes Simplex Virus (HSV)
Herpes Simplex Virus-2 (HSV-2) is the common cause of most of genital herpes simplex infections worldwide with the CDC estimating that 50 million people in the U.S. were infected with HSV-2 in 2015 (Workowski & Bolan, 2015). More than 770,000 people in the U.S. are infected each year with genital herpes; moreover, HSV-1 genital herpes has increased in recent years. This trend is believed to be due to a decline in childhood oral HSV-1 infections that in the past increased immune resistance to genital HSV-1 infections (CDC, 2021b). Primary genital herpes infections can present with genital ulcers as well as other immunological responses, such as fever and lymphadenopathy; however, for some people, a primary genital herpes infection is asymptomatic. Nonprimary infections occur when a patient acquires HSV-1 with pre-existing HSV-2 antibodies or vice versa. Recurrent infections can be either symptomatic or asymptomatic, which can be referred as subclinical. A minority of HSV-positive patients can also present with meningitis and/or proctitis (Albrecht, 2020). Vertical transmission from mother to newborn can occur during delivery, especially if the mother acquires a primary infection near the end of the pregnancy. This vertical transmission can occur even if the mother is asymptomatic (Riley & Wald, 2022). Diagnosis of genital herpes infection can be performed by viral culture, NAAT, and serological testing. “Cell culture and PCR-based testing are the preferred tests for a patient presenting with active lesions, although PCR-based testing has the greatest overall sensitivity and specificity” (Albrecht, 2020).
Human Papillomavirus (HPV)
Anogenital HPV infection is the most common STI worldwide with an estimation that “at least 80 percent of sexually active women and men are exposed to HPV once in their lifetime. However, many experts believe that virtually all sexually active adults have been infected by HPV…”(Palefsky, 2022). This is due to the large number of different types of HPV known to infect the genital tract — at least 40 characterized to date — and the transitory nature of HPV infections. HPV is associated with a variety of cancers, including anal, penile, vulvar, vaginal, and oropharyngeal cancer; moreover, the carcinogenic effect of an HPV infection can be years after the initial diagnosis of HPV. Multiple HPV vaccinations have been approved for use in the U.S., and the CDC recommends vaccination for HPV for all children ages 11 or 12 (CDC, 2021c). HPV can be detected from swab samples and can be included in many routine cervical exams. High-risk oncogenic HPV testing is commercially available (Feldman & Crum, 2022).
HIV Preexposure Prophylaxis (PrEP)
An estimated 1.1 million people in the United States currently live with human immunodeficiency virus (HIV). HIV is a virus that, while treatable, does not have a cure and results in serious health consequences that may include acquiring AIDs (acquired immune deficiency syndrome). In the 2019 issue of JAMA, the US Preventive Services Task Force updated guidelines on recommendations for HIV screening and preventive services. The USPSTF reviewed the evidence regarding Preexposure prophylaxis (PrEP), which is the use of antiretroviral medication to prevent HIV infection ang provided a grade A recommendation for PrEP in certain circumstances (CDC, 2021e; USPSTF, 2019). The USPSTF determined that PrEP is “of substantial benefit in decreasing the risk of HIV infection in persons at high risk of HIV acquisition” (USPSTF, 2019). As a preventive medication, PrEP involves a single treatment taken orally with “combined tenofovir disoproxil fumarate and emtricitabine,” or tenofovir disoproxil fumarate alone, which can be considered as an alternative regimen (USPSTF, 2019). In addition, adherence to PrEP is “highly associated with its efficacy in preventing the acquisition of HIV infection; thus, adherence to PrEP is central in realizing its benefit.” Overall, the guidance is to provide PrEP with antiretroviral therapy to persons at high risk of HIV acquisition (USPSTF, 2019).
To determine status for PrEP provision, the CDC recommends antigen/antibody testing to confirm that patients do not currently have HIV infection. At a minimum providers should test to confirm a negative antibody result within a week before initiating (or re-initiating) PrEP regimens (CDC, 2021e). There are a few ways to accomplish HIV testing: “(1) drawing blood and sending the specimen to a laboratory for testing or (2) performing a rapid, point-of-care FDA-approved fingerstick blood test. Oral rapid tests should not be used to screen for HIV infection when considering PrEP use because they can be less sensitive than blood tests” (CDC, 2021e).
The PrEP regimen may cause decreases in renal function. Usually, these are of small or limited clinical significance, but occasional cases of acute renal failure have been documented. The CDC guidance indicates that all patients who are considered for PrEP should have renal function assessed during the beginning of treatment. Other screenings recommended before PrEP initiation include a screening for HBV.
The following table for PrEP testing recommendations for clinicians was compiled by the CDC (CDC, 2021e):
|
Provide the following services: |
Screening tests/samples |
|
At 3 months after PrEP initiation: |
• Test for HIV. • Measure serum creatinine and estimate creatinine clearance. • Provide medication adherence and behavioral risk reduction support. • Additionally, for o MSM: screen for bacterial STIs*; o Women with reproductive potential: test for pregnancy; and o PWID: assess access to sterile needles/syringes and to drug treatment services. |
|
Every 3 months after the first 3-month follow-up |
• Test for HIV. • Provide medication adherence and behavioral risk reduction support. • Additionally, for o MSM: screen for bacterial STIs*; o Women with reproductive potential: test for pregnancy; and o PWID: assess access to sterile needles/syringes and to substance use disorder treatment services. |
|
Every 6 months after the first 3-month follow-up |
• Measure serum creatinine and estimate creatinine clearance. • For all sexually active patients: Screen for bacterial STIs*. |
*Nucleic Acid Amplification Test (NAAT) to screen for gonorrhea and chlamydia based on anatomic site of exposure; blood test for syphilis.
Proprietary Testing
BD Onclarity HPV Assay
The BD Onclarity HPV Assay, a qualitative in vitro assay of cervical swabs using PCR (i.e., a nucleic acid amplification test or NAAT), is offered by Becton, Dickinson and Company and is approved by the FDA. This test specifically identifies types 16, 18 and 45, while concurrently detecting the other high-risk (HR) HPV types (including 31, 51, 52, 33/58, 35/39/68, and 56/59/66). For HR-HPV 31, 51, 52, 33/58, 35/39/68, and 56/59/66, this is “the only FDA-approved assay to individually identify and report these genotype results” (BD, 2020).
Becton, Dickinson and Company note that “the BD Onclarity HPV Assay is indicated: 1) In women 21 years and older with ASC-US (atypical squamous cells of undetermined significance) cervical cytology test results, the BD Onclarity HPV Assay can be used to determine the need for referral to colposcopy; 2) In women 21 years and older with ASC-US cervical cytology test results, the BD Onclarity HPV assay can be used to detect high-risk HPV genotypes 16, 18 and 45. This information together with physicians assessment of screening history, other risk factors, and professional guidelines, may be used to guide patient management. The results of this test are not intended to prevent women from proceeding to colposcopy; 3) In women 30 years and older, the BD Onclarity HPV Assay can be used together with cervical cytology to adjunctively screen to detect high risk HPV types. This information, together with the physicians assessment of screening history, other factors, and professional guidelines, may be used to guide patient management; 4) In women 30 years and older, the BD Onclarity HPV Assay can be used to detect high-risk HPV genotypes 16, 18 and 45. This information, together with the physicians assessment of screening history, other factors, and professional guidelines, may be used to guide patient management; and 5) In women 25 years and older, the BD Onclarity HPV Assay can be used as a first-line primary cervical cancer screening test to detect high risk HPV, including 16 and 18. Women who test negative for the high risk HPV types by the BD Onclarity HPV Assay should be followed up in accordance with the physicians assessment of screening and medical history, other risk factors, and professional guidelines. Women who test positive for HPV genotypes 16 and/or 18 by the BD Onclarity HPV Assay should be referred to colposcopy. Women who test high risk HPV positive and 16 and 18 negative by the BD Onclarity HPV Assay (12 other HR HPV Positive) should be evaluated by cervical cytology to determine the need for referral to colposcopy (FDA, 2021).”
Cepheid Xpert® CT/NG
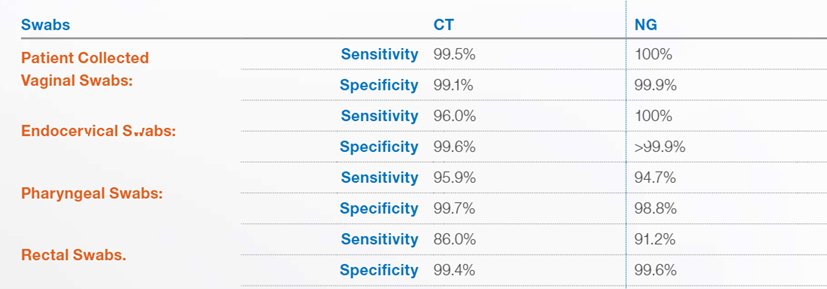
Cepheid offers the Cepheid Xpert® CT/NG test, an FDA approved nucleic acid amplification test to detect Chlamydia trachomatis (CT) and/or Neisseria gonorrhoeae (NG) using urogenital specimens and extragenital specimens (pharynx and rectum))(FDA, 2012a, 2019a). It is performed using the GeneXpert® Instrument Systems with a qualitative in vitro real-time PCR “for the automated detection and differentiation of genomic DNA from Chlamydia trachomatis (CT) and/or Neisseria gonorrhoeae (NG)” (FDA, 2012b, 2019b) and is stated to provide results for up to 96 specimens in approximately 90 minutes (Cepheid, 2022b). The assay may be used to “test the following specimens from asymptomatic and symptomatic individuals: female and male urine, patient-collected vaginal swabs (collected in a clinical setting), clinician-collected endocervical swabs, and female and male pharyngeal and rectal swabs” (Cepheid, 2022b).
The test has varying sensitivities and specificities based on the sample obtained and for which disease the assay is testing for. They are listed below (Cepheid, 2022a):
Analytical Validity
A 2005 study by Cook and colleagues (Cook et al., 2005) reviewed the validity of NAAT for chlamydia and gonorrhea from urine samples as compared to swabs obtained directly from either the cervix or urethra. They reviewed 29 different studies and only included studies using collections of samples obtained from two anatomic sites. Each test required either a secondary culture confirmation or a secondary NAAT-based confirmation. Over 20,000 different patients were included in the pooled study, and three different NAAT assays were monitored — polymerase chain reaction (PCR), transcription-mediated amplification (TMA), and strand displacement amplification (SDA). “The pooled study specificities of each of the 3 assays exceeded 97% when urine samples were tested, for both chlamydial infection and gonorrhea and in both men and women.” The use of PCR for gonorrheal testing, though, from female urine samples had only 55.6% specificity. The authors concluded the following: “Results of nucleic acid amplification tests for C. trachomatis on urine samples are nearly identical to those obtained on samples collected directly from the cervix or urethra. Although all 3 assays can also be used to test for N. gonorrhoeae, the sensitivity of the polymerase chain reaction assay in women is too low to recommend its routine use to test for gonorrhea in urine specimens (Cook et al., 2005).”
Due to an increase in demand for enzyme immunoassay-based testing of syphilis, Wong et al. (2011) evaluated the validity of such testing — using the Trep-Sure EIA test — to that of the documented Venereal Disease Research Laboratory (VDRL) test and Treponema pallidum particle agglutination (TPPA) assay. Their research included 674 samples. The EIA-based test had a sensitivity of 98.0% and a specificity of 98.6% (Cantor et al., 2016). The authors conclude that “an IgM/IgG sensitive EIA would be an effective alternative to VDRL for syphilis screening” (Wong et al., 2011). An earlier study using another EIA-based assay, the Trep-Check IgG EIA test, conducted at the National Microbiology Laboratory of Canada (Tsang et al., 2007) did not report as positive results as the Wong study. This research consisted of 604 samples submitted from local or provincial hospitals for confirmation of local testing. Their findings were that the Trep-Check IgG EIA had a sensitivity of 85.3% and specificity of 95.6%, but they also report a positive predictive value of 53.7% (Tsang et al., 2007) as compared to the positive predictive value of 98.4% of the Trep-Sure EIA test (Cantor et al., 2016; Wong et al., 2011). These results can be compared to the published results of the accuracy of the TPPA assay of 87.1% sensitivity, 100% specificity, and 100% positive predictive value — albeit in a smaller sample size (n = 198) (Cantor et al., 2016; Juarez-Figueroa et al., 2007).
The US Preventive Services Task Force (USPSTF) conducted a systematic review of the use of serologic screening for genital herpes and published their findings in 2016 (Feltner et al., 2016). Their extensive review consisted of 17 different studies, ranging from 24 to 3,290 participants, in 19 different publications. Reviewing only the serological testing of HSV-2, they note that the “pooled estimates of sensitivity and specificity of the most commonly used test at the manufacturer’s cutpoint were 99% (95% CI, 97% – 100%) and 81% (95% CI, 68% – 98%), respectively.” However, they also note that “use of this test at the manufacturer’s cutpoint in a population of 100 000 with a prevalence of HSV-2 of 16% (the seroprevalence in U.S. adults with unknown symptom status) would result in 15 840 true-positive results and 15,960 false-positive results (positive predictive value, 50%).” They note the potential psychosocial harm due to false-positive results. The authors conclude, “Serologic screening for genital herpes is associate with a high rate of false-positive test results and psychosocial harms” (Feltner et al., 2016).
In 2021, the U.S. Preventive Services Task Force issued a brief update on genital herpes simplex diagnostics. Their assessment found that viral culture continues to be the gold standard for HSV infections. For central nervous system infections of HSV, PCR continues to be the gold standard, because of the assay’s sensitivity of 80% to 90% for lesion specimens. They also indicated that serological tests are used to detect previous infections of herpes simplex in asymptomatic patients, specifying the Western blot assay as the most validated method. In addition, they noted: “two type-specific glycoprotein G serological tests are commercially available in the United States. Sensitivity and specificity of these tests are comparable to the Western blot assay” (Glass, 2021).The ATHENA study conducted in 2008 – 2009 and published in Lancet in 2011 consisted of more than 40,000 women in the U.S. aged 25 or over in 61 different clinical centers. The goal was to assess high-risk HPV16 and HPV18 testing versus traditional methods. Their results show that “in women who had colposcopy, the Cobas HPV test was more sensitive than liquid-based cytology for detection of CIN3 [cervical intraepithelial neoplasia grade 3] or worse” with 92.0% versus 53.3% for liquid cytology. “Addition of liquid-based cytology to HPV testing increased sensitivity for CIN3 or worse to 96.7% ... but increased the number of screen positives by 35.2%.” The authors conclude, “HPV testing with separate HPV16 and HPV18 detection could provide an alternative, more sensitive, and efficient strategy for cervical cancer screening than do methods based solely on cytology (Castle et al., 2011).” Guenat and colleagues report a coefficient of variation of less than 8% for repeatability and reproducibility when using the Novaprep HQ+ medium in liquid-based cytology for HPV (Guenat et al., 2016). Another study comparing the validity of using urine samples in comparison with cervical samples for monitoring HPV in women over the age of 30 shows that the sensitivity of the urine testing varies considerably depending on the NAAT assay used. The multiplex type-specific PCR (E7-MPG) assay had a sensitivity of 80% and specificity of only 61% whereas the GP5+/6+ PCR assay resulted in 58% and 89%, respectively, for sensitivity and specificity as compared to the gold standard cervical swabs (Tshomo et al., 2017).
A study by Golden et al. (2019) compared the sensitivity of syphilis serological testing using the rapid plasma reagin (RPR) test and an experimental 23S rRNA Treponema pallidum real-time transcription-mediated amplification (TMA) assay. This study included 545 men who have sex with men (MSM); a total of 506 pharyngeal specimens and 410 rectal specimens were provided for this study. Twenty-two men were diagnosed with syphilis based on serological testing results; further, two more men were diagnosed based on TMA testing results. The authors report that “At least 1 specimen was TMA positive for 12 of 24 men with syphilis (sensitivity, 50% [95% confidence interval [CI], 29 to 71%]). RPR testing and clinical diagnosis were 92% sensitive (95% CI, 73 to 99%) in identifying infected men” (Golden et al., 2019). A combinatory approach of mucosal TMA testing and serological testing may improve the sensitivity of syphilis screening.
Pham et al. (2020) reported on a new prototype POCT based on detecting IgA antibodies for Treponema pallidum (TP-IgA), which is a new biomarker for active syphilis. Using “458 pre-characterised stored plasma in China … and 503 venous blood samples collected from pregnant/postpartum in South Africa,” the performance of the POCT was compared against TPHA and RPR tests. In the sub-study group from China, the index test had a sensitivity of 96.1% (95% confidence interval 91.7% – 98.5%) and specificity of 84.7% (95% confidence interval 80.1% – 88.6%) for “identification of active syphilis,” (TPHA positive, RPR positive) and identified 71% samples of past-treated syphilis, defined as a TPHA positive but RPR negative test. In the sub-study group from South Africa, the index test had a 100% sensitivity (95% confidence interval 59% – 100%) for active syphilis, and “correctly identified all nine women with past syphilis.” The researchers cite that in comparison to other POCTs on the market, this new test can “identify past syphilis whilst maintaining a high sensitivity for active syphilis infections,” and “support[s] the global effort in prevention of mother to child transmission and elimination of congenital syphilis in settings where laboratory capacity is limited” (Pham et al., 2020).
In 2019, Bristow et al. compared the use of the Xpert® CT/NG test on extragenital samples to the already FDA-approved APTIMA transcription mediated amplification Combo 2 assay. They found the Xpert® CT/NG test performed similarly, but with a faster turnaround time and increased potential for same-day treatment. Their results demonstrated that “the pooled positive and negative percent agreement for detection of CT in rectal specimens was 89.72% (95% CI: 84.97%, 93.64%) and 99.23% (95% CI: 98.74%, 99.60%), and in pharyngeal specimens, they were 89.96% (95% CI: 66.38%, 99.72%) and 99.62% (95% CI: 98.95%, 99.95%) respectively. For NG detection in rectal specimens, the pooled positive and negative per cent agreement was 92.75% (95% CI: 87.91%, 96.46%) and 99.75% (95% CI: 99.46%, 99.93%), and in pharyngeal specimens, they were 92.51% (95% CI: 85.84%, 97.18%) and 98.56% (95% CI: 97.69%, 99.23%) respectively” (Bristow et al., 2019).
A separate study done earlier by Cosentino et al. (2017) also compared APTIMA’s transcription mediated Combo 2 assay with the Xpert® CT/NG assay and found that “For C. trachomatis, neither system was > 95% sensitive from the rectum, though both were > 99.5% specific. For N. gonorrhoeae, Xpert had higher sensitivity than Aptima, but with more false positives from pharyngeal samples.”
Clinical Validity and Utility
A 2017 review of point-of-care tests (POCTs) versus near-patient NAAT for chlamydia reviewed 11 different studies consisting of a combined total of more than 13,000 patients. The pooled results show that POCTs have a sensitivity of only 53%, 37%, and 63% for cervical swabs, vaginal swabs, and male urine, respectively, but that the specificity for each ranged from 97 – 99%. The near-patient NAAT has a sensitivity of > 98% regardless of sample with a specificity of 99.4%. “The systematic reviews show that antigen detection POCTs for CT [C. trachomatis], although easy to use, lacked sufficient sensitivity to be recommended as a screening test. A near-patient NAAT shows acceptable performance as a screening or diagnostic test but requires electricity, takes 90 min and is costly (Kelly et al., 2017).” Likewise, a review of five POCTs and one near-patient NAAT for gonorrhea in 2017 show that POTC immunochromatographic tests and optical immunoassays had sensitivities ranging from 12.5% to 70% compared to laboratory NAAT for cervical and vaginal swab samples. The specificities of the near-patient NAATs were > 99.8% with sensitivities > 95% (Guy et al., 2017).
A 2018 review of laboratory testing for T. pallidum in Australia (Brischetto et al., 2018) compared the clinical value of PCR testing for syphilis as compared to the traditional serological testing using RPR, agglutination, and/or chemiluminescence immunoassay (CMIA). This review covered all testing at the Australian lab from 2010 to 2017. They show that 19% of PCR results were positive for syphilis with 97% of those patients also showing positive serological results. The T. pallidum PCR had a sensitivity of 68% and specificity of 99% as compared to the serology testing sensitivity of 97% and 88% specificity. “Our results show that most patients with positive T. pallidum PCR results also had positive syphilis serology. Therefore, T. pallidum PCR adds little clinical value over serology for the diagnosis of syphilis in certain clinical settings (Brischetto et al., 2018).” A 2015 Chinese study (Zhiyan et al., 2015) does show that the CMIA screening is not as specific as the TPPA agglutination assay for syphilis with 18 of the 149 CMIA-positive samples being false-positive results.
The 2016 USPSTF review of genital herpes serological testing (Feltner et al., 2016) included a review of the HerpeSelect serological test consisting of the data from ten studies with a combined total of 6537 participants. The pooled, combined results show a sensitivity of 99% and specificity of 81%. Four additional studies they reviewed used the biokit HSV-2 Rapid Test assay. These studies had a combined total of 1512 participants. The sensitivity is considerably lower (84%), but the specificity was higher than the HerpeSelect assay (95%).
A study by Liu and associates (Liu et al., 2014) evaluated the clinical performance of the QuantiVirus HPV E6/E7 mRNA with respect to identifying ≥ Grade 2 cervical intraepithelial neoplasia. Approximately 40.3% of the 335 female patients tested positive for high-risk HPV. They note that “the positivity rate of HPV E6/E7 mRNA increased with the severity of cytological and histological evaluation … a high specificity and a low positivity rate of E6/E7 mRNA testing as a triage test in HPV DNA-positive women can be translated into a low referral for colposcopy (Liu et al., 2014).” Another study of the QuantiVirus system in 2017 (Yao et al., 2017) of 404 HPV-positive women show no statistical difference between QuantiVirus and cytological testing in sensitivity, specificity, positive predictive value, and negative predictive value for predicting high-grade squamous intraepithelial lesion (HSIL). “HPV E6/E7 mRNA detection in cervical exfoliated cells shows the same performance as Pap triage for HSIL identification for HPV-positive women. Detection of HPV E6/E7 mRNA may be used as a new triage option for HPV-positive women (Yao et al., 2017).” A review by Arbyn and colleagues concerning the efficacy of repeat cytology versus HPV testing for atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesions (LSIL) demonstrated that the pooled sensitivity of the Hybrid Capture 2 (HC2) assay for the high-risk HPV types was significantly higher than performing repeat cytology (relative sensitivity of 1.27 and 1.23, respectively) for detecting CIN2+ but was significantly lower than repeat cytology for LSIL. “HPV-triage with HC2 can be recommended to triage women with ASCUS because it has higher accuracy…than repeat cytology. When triaging women with LSIL, an HC2 test yields a significantly higher sensitivity, but a significantly lower specificity, compared to repeat cytology. Therefore, practice recommendations for management of women with LSIL should be balanced, taking local circumstances into account (Arbyn et al., 2013).”
A study by Gaydos et al. (2019) showed that, for women in the emergency department (ED), the use of rapid diagnostic tests for Chlamydia trachomatis and Neisseria gonorrhoeae infections can improve clinical management. This randomized clinical trial was composed of 254 women undergoing pelvic examinations for both C. trachomatis and N. gonorrhoeae testing; the women were split into control and rapid test groups. For the rapid test group, the GeneXpert rapid test was used. The authors report that “Undertreatment for both C trachomatis and N gonorrhoeae in the ED was 0% for the rapid test group and 43.8% for the control standard-of-care group. Clinicians overtreated 46.5% of uninfected standard-of-care control patients for C trachomatis compared with 23.1% of uninfected rapid test patients. For patients uninfected with N gonorrhoeae, clinicians overtreated 46.7% of standard-of-care control patients compared with 25.4% of rapid test patients” (Gaydos et al., 2019). These results show that rapid testing of C. trachomatis and N. gonorrhoeae led to a significant reduction in overtreatment compared to the control group.
National Comprehensive Cancer Network (NCCN)
Anal Carcinoma (NCCN, 2022a): HPV, especially high-risk types HPV-16 and HPV-18, are linked to anal carcinoma. The NCCN refers to a study that detected HPV in 84% of anal carcinoma samples and 0% in rectal cancer samples, and they state that “the prevalence of HPV-16/18 to be 72% in patients with invasive anal cancer.” Precursor high-grade anal intraepithelial neoplasia (AIN) “can be identified by cytology, HPV testing, digital rectal examination (DRE), high-resolution anoscopy, and/or biopsy.” They also state that “data suggest that HPV- and/or p16-positivity are prognostic for improved OS [overall survival] in patients with anal carcinoma.” For females, the NCCN also recommends a gynecologic examination, including cervical cancer screening, due to the link between HPV and anal carcinoma.
Cervical Cancer (NCCN, 2021a): “Persistent human papillomavirus (HPV) infection is the most important factor in the development of cervical cancer. The incidence of cervical cancer appears to be related to the prevalence of HPV in the population…. Screening methods using HPV testing may increase detection of adenocarcinoma.” The NCCN lists chronic, persistent HPV infection along with persistently abnormal Pap tests as criteria to be considered for women contemplating hysterectomy after the completion of childbearing.
Head and Neck Cancers (NCCN, 2022b): The NCCN in the Head and Neck Cancers guidelines now specifically states, “Tumor human papillomavirus (HPV) testing by p16 immunohistochemistry (IHC) required” in their workup for cancer of the oropharynx because the p16 status dictates the treatment options to be considered (per the ORPH-1 workup). This version of the guidelines also includes a page on the “Principles of P16 Testing for HPV-Mediated Oropharyngeal Cancer” where they state the following:
- “P16 expression is highly correlated with HPV status and prognosis and is widely available.”
- “A few HPV testing options are available for use in the clinical setting. Expression of p16 as detected by IHC is a widely available surrogate biomarker that has very good agreement with HPV status as determined by the gold standard of HPV E6/E7 mRNA expression. Other tests include HPV detection through PCR and in situ hybridization (ISH).
- “Sensitivity of IHC staining for p16 and PCR-based assay is high, although specificity is highest for ISH.”
- “Due to variations in sensitivity and specificity values of testing options, multiple methods may be used in combination for HPV detection, but HPV detection through PCR and ISH may provide additional sensitivity for the former and specificity for the latter in the case of an equivocal p16 or unclear clinical scenario.”
- “Sufficient pathologic material for HPV testing can be obtained through FNA.”
- “A small proportion of tumors at non-oropharyngeal sites (e.g., paranasal sinus, oral cavity, larynx) are HPV-related. However, given the small proportion and lack of consistent evidence in support of prognostic significance, routine HPV testing or p16 [testing] of non-oropharyngeal cancers is not recommended.”
- “Guidelines for testing are available from the College of American Pathologists.”
Occult Primary Cancers (NCCN, 2021c): The NCCN now lists HPV to be tested for Occult Primary cancers. The NCCN also states that for squamous cell carcinoma with a clinical presentation in the head and neck nodes, “Check results of p 16 immunohistochemistry/HPV in situ hybridization and EBV in situ hybridization; positive results can help localize primary site.” Further, the guidelines note that HPV can be used as a potential immunohistochemistry marker for unknown primary cancers, including tumors identified in the cervix, vulva, vagina, penis, anal, oropharynx; a nuclear (DNA ISH) or nuclear/cytoplasmic (RNA ISH) staining pattern is recommended (NCCN, 2021c).
Penile Cancer (NCCN, 2022c): “Overall, approximately 45% to 80% of penile cancers are related to HPV, with a strong correlation with types 16, 6 and 18.” Discerning whether a penile cancer lesion is infected with HPV is important for laser ablation therapy as noted in the section titled “Principles of Penile Organ-Sparing Approaches.”
Vulvar Cancer (NCCN, 2021b): “Risk factors for the development of vulvar neoplasia include increasing age, infection with human papillomavirus (HPV), cigarette smoking, inflammatory conditions affecting the vulva, and immunodeficiency…. Usual-type VIN [vulvar intraepithelial neoplasia] was linked to persistent infection with carcinogenic strains of HPV, while differentiated VIN was commonly associated with vulvar dermatologic conditions such as lichen sclerosus. In 2015, the ISVVD updated the description to three classes of vulvar lesions: 1) low-grade squamous intraepithelial lesion (LSIL) due to flat condyloma or HPV effect; 2) high-grade squamous intraepithelial lesions (HSIL, formerly considered usual-type VIN); and 3) differentiated VIN.” The NCCN notes that 80 – 90% of HSIL cases have HPV infections, and that between 30% – 69% of all vulvar cancers are believed to be “attributable to HPV infection.” In the “Diagnosis and Workup” section, they state, “Appropriate patients should receive smoking cessation counseling and HPV testing.” The guidelines also note for the surveillance of vulvar cancer: “cervical/vaginal cytology screening as indicated for the detection of lower genital tract neoplasia (may include HPV testing)” (NCCN, 2021b).
U.S. Preventive Services Task Force (USPSTF)
Screening for Chlamydia and Gonorrhea (Davidson et al., 2021): The USPSTF recommends (Grade B) to screen for chlamydia and gonorrhea in “sexually active females aged 24 years or younger and in women 25 years or older who are at increased risk for infection.” They also conclude (an “I” statement) “that the current evidence is insufficient to assess the balance of benefits and harms of screening for chlamydia and gonorrhea in men.” Besides age, “women 25 years or older are at increased risk for infection if they have a new sex partner, more than 1 sex partner, a sex partner with concurrent partners, or a sex partner who has an STI; practice inconsistent condom use when not in a mutually monogamous relationship; or have a previous or coexisting STI. Exchanging sex for money or drugs and history of incarceration also are associated with increased risk.” They clearly state that both chlamydia and gonorrhea should be tested using NAATs.
Screening for Oral Cancer (Moyer, 2014): Given the link between HPV infection and oral cancers, the USPSTF released their findings concerning the screening of asymptomatic patients. “The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for oral cancer in asymptomatic adults.” They also state the following: “Although there is interest in screening for oral HPV infection, medical and dental organizations do not recommend it. Currently, no screening test for oral HPV infection has been approved by the U.S. Food and Drug Administration (FDA). Evaluating the accuracy of tests that detect oral HPV infection is a potentially promising area of research (Moyer, 2014).”
Serological Screening for Genital Herpes (Feltner et al., 2016): HSV-2 is the primary causative agent of genital herpes, and HSV-2 infection during pregnancy can cause fetal morbidity and mortality. Due to its prevalence in the U.S. and the possible consequences of a genital herpes infection, the USPSTF researched the validity and practicality of HSV-2 screening in asymptomatic patients. They conclude that “serologic screening for genital herpes is associated with a high rate of false-positive test results and potential psychosocial harms. Evidence from RCTs [randomized clinical trials] does not establish whether preventive antiviral medication for asymptomatic HSV-2 infection has benefit.” Overall, the USPSTF “recommends against routine serologic screening for genital herpes simplex virus (HSV) infection in asymptomatic adolescents and adults, including those who are pregnant.”
Screening for Syphilis (Cantor et al., 2016): Previously, in 2004, the USPSTF “recommended routine screening for syphilis in asymptomatic men and nonpregnant women at increased risk of infection (A recommendation) and recommended against routine screening for those not at increased risk (D recommendation).” The previous study did not address the frequency of repeat testing. The current 2016 study adds to the previous recommendations. “Screening HIV-positive men or MSM for syphilis every 3-months is associated with improved syphilis detection. Treponemal or nontreponemal tests are accurate screening tests but require confirmation. Research is needed on the effect of screening on clinical outcomes; effective screening strategies, including reverse sequence screening, in various patient populations; and harms of screening.”
Centers for Disease Control and Prevention (CDC)
Diseases Characterized by Genital, Anal, or Perianal Ulcers: “… all persons who have genital, anal, or perianal ulcers should be evaluated; . . . Specific evaluation of genital, anal, or perianal ulcers includes syphilis serology tests and darkfield examination from lesion exudate or tissue, or NAAT if available; NAAT or culture for genital herpes type 1 or 2; and serologic testing for type-specific HSV antibody. In settings where chancroid is prevalent, a NAAT or culture for Haemophilus ducreyi should be performed.” Later, in the section specifically focused on genital HSV infections, the CDC states, “Both type-specific virologic and type-specific serologic tests for HSV should be available in clinical settings that provide care to persons with or at risk for STIs.” They stress that the patient’s prognosis does depend on the type of HSV infection, especially since “recurrences and subclinical shedding are much more frequent for genital HSV-2 infection than for genital HSV-1 infection.” Regarding testing, “HSV NAAT assays are the most sensitive tests because they detect HSV from genital ulcers or other mucocutaneous lesions; these tests are increasingly available”(CDC, 2021f). NAATs are more sensitive than viral culture testing. On the CDC’s detailed fact sheet about genital herpes, they state, “Routine serologic HSV screening of pregnant women is not recommended” (CDC, 2021b).
In guidance on serology, the CDC states in 2021 that “type-specific HSV-2 serologic assays for diagnosing HSV-2 are useful in the following scenarios: recurrent or atypical genital symptoms or lesions with a negative HSV PCR or culture result, clinical diagnosis of genital herpes without laboratory confirmation, and a patient’s partner has genital herpes. HSV-2 serologic screening among the general population is not recommended. Patients who are at higher risk for infection (e.g., those presenting for an STI evaluation, especially for persons with ≥ 10 lifetime sex partners, and persons with HIV infection) might need to be assessed for a history of genital herpes symptoms, followed by type-specific HSV serologic assays to diagnose genital herpes for those with genital symptoms”(CDC, 2021f).
Syphilis: Darkfield examinations and molecular tests for detecting T. pallidum lesion cells, fluid, or tissue are the gold standard methods for diagnosing early syphilis and congenital syphilis. According to the CDC, “Although no T. pallidum direct detection molecular NAATs are commercially available, certain laboratories provide locally developed and validated PCR tests for detecting T. pallidum DNA. A presumptive diagnosis of syphilis requires use of two laboratory serologic tests: a nontreponemal test (i.e., Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR] test) and a treponemal test (i.e., the T. pallidum passive particle agglutination [TP-PA] assay, various EIAs, chemiluminescence immunoassays [CIAs] and immunoblots, or rapid treponemal assays) … Use of only one type of serologic test (nontreponemal or treponemal) is insufficient for diagnosis and can result in false-negative results among persons tested during primary syphilis and false-positive results among persons without syphilis or previously treated syphilis.” If a patient shows signs and symptoms of neurosyphilis, including “cranial nerve dysfunction, auditory or ophthalmic abnormalities, meningitis, stroke, acute or chronic altered mental status, and loss of vibration sense,” further testing is required-CSF cell count or protein and a reactive CSF-VDRL (CDC, 2021f).
Chlamydial Infections: “Annual screening of all sexually active women aged < 25 years is recommended, as is screening of older women at increased risk for infection (e.g., those who have a new sex partner, more than one sex partner, a sex partner with concurrent partners, or a sex partner who has a sexually transmitted infection…screening of sexually active young men should be considered in clinical settings with a high prevalence of chlamydia (e.g., adolescent clinics, correctional facilities, or STD specialty clinics) or for populations with a high burden of infection (e.g., MSM)” (CDC, 2021f).
NAAT testing of first-catch urine or swab specimens is recommended. In the diagnostic considerations section of Chlamydial Infections, the CDC does not address any differences between symptomatic or asymptomatic screening, and they do not mention any specific diagnostic considerations of patients showing signs or symptoms of a chlamydial infection. In the 2014 CDC guide for laboratory testing of chlamydia and gonorrhea, they, too, recommend using NAATs and not the older nonculture or non-NAAT testing methods. For extragenital infections such as rectal and oropharyngeal infections, the CDC recommends testing at the anatomic exposure site. NAATs demonstrate improved sensitivity and specificity in comparison to culture for extragenital infection. In addition, newly available molecular point-of-care (POC) tests for asymptomatic persons can help with faster, more efficient treatment. With symptomatic cases these POC tests can also “optimize treatment by limiting unnecessary presumptive treatment at the time of clinical decision-making and improve antimicrobial stewardship. Thus, using a POC test will likely be a cost-effective diagnostic strategy for C. trachomatis infection … newer NAAT-based POC tests have promising performance and are becoming commercially available” (CDC, 2021f).
Gonococcal Infections: The CDC recommendation concerning gonococcal screening is similar to that of chlamydia — sexually active women aged < 25 years and older women and men in high-risk categories. “Screening for gonorrhea in men and older women who are at low risk for infection is not recommended” (CDC, 2021f). For testing genitourinary infection with N. gonorrhoeae, “culture, NAAT, and POC NAAT, such as GeneXpert (Cepheid), are available.” NAAT allows for best testing of genitourinary infection.
For rectal, oropharyngeal, and conjunctival infections, culture is available. The CDC states that “NAATs and POC NAATs allow for the widest variety of FDA-cleared specimen types, including endocervical and vaginal swabs and urine for women, urethral swabs and urine for men, and rectal swabs and pharyngeal swabs for men and women. However, product inserts for each NAAT manufacturer should be consulted carefully because collection methods and specimen types vary. Certain NAATs that have been demonstrated to detect commensal Neisseria species might have comparable low specificity when testing oropharyngeal specimens for N. gonorrhoeae. NAAT sensitivity for detecting N. gonorrhoeae from urogenital and nongenital anatomic sites is superior to culture but varies by NAAT type. NAAT testing of rectal and/or oropharyngeal swab specimens can be performed in certain laboratories that have met CLIA requirements even though the testing methodology has not been FDA-approved”(CDC, 2021f). Follow-up testing post-treatment for urogenital or rectal gonorrhea is not necessary, but NAAT testing should be performed 14 days after treatment for pharyngeal gonorrhea. Vaginitis is the most common symptom of infection in preadolescent girls (Workowski & Bolan, 2015).”
In the 2014 laboratory guide, the CDC states that “N. gonorrhoeae culture capacity is still needed for evaluating suspected cases of treatment failure and monitoring antimicrobial susceptibility.” They also state, “C. trachomatis and N. gonorrhoeae culture capacity might still be needed in instances of child sexual assault in boys and extragenital infections in girls” (Papp et al., 2014).
Human Papillomavirus Infections: Even though testing for oncogenic HPV variants exists, the CDC states, “These tests should not be used for male partners of women with HPV or women aged < 25 years, for diagnosis of genital warts, or as a general STI test.” For patients showing signs and symptoms of anogenital warts, the CDC states, “HPV testing is not recommended for anogenital wart diagnosis, because test results are not confirmatory and do not guide genital wart management.” For cervical screening, “For persons aged 30 – 65 years, a cytology test every 3 years, an HPV test alone every 5 years, or a cytology test plus an HPV test (cotest) every 5 years is recommended” (CDC, 2021f).
The CDC (2021c) also notes that “Routine screening for women aged 21 to 65 years old can prevent cervical cancer”; further, “There are HPV tests that can be used to screen for cervical cancer. Healthcare providers only use these tests for screening in women aged 30 years and older. HPV tests are not recommended to screen men, adolescents, or women under the age of 30 years.”
Finally, the CDC (2022a) states that “there is currently no approved test for HPV in men. CDC does not recommend routine testing (also called ‘screening’) for HPV in men. CDC also does not recommend routine testing for diseases from HPV before there are signs or symptoms in men. Some healthcare providers offer anal Pap tests to men who may be at greater risk for anal cancer. This includes men with HIV or men who receive anal sex. If you have symptoms and are concerned about cancer, please see a healthcare provider.”
International Union Against Sexually Transmitted Infections (IUSTI)
The Management of Anogenital Warts (European): “HPV detection or typing does not influence management and is not recommended. Some practitioners use the acetic acid test to diagnose sub-clinical HPV lesions; its place in diagnosis and management is uncertain” (Gilson et al., 2020) .
The Diagnosis and Treatment of Gonorrhea in Adults (Unemo, 2020) NAATs, bacterial culture, and microscopy can be used in the diagnosis of uncomplicated gonorrhea. “No test offers 100% sensitivity and specificity.” They do state (with a grade C recommendation) that microscopy can be used for testing symptomatic men, but it is not recommended for use in asymptomatic men, rectal infection, or endocervical infection due to low sensitivity. Culture testing is the only method to use for determining antimicrobial susceptibility, but culture testing is not as sensitive as NAAT. For NAAT-based point-of-care tests (POCTs), the guideline says: “several NAAT-based POCTs with high sensitivity and specificity are in late development.” The IUSTI includes the following list for “Indications for testing” (grade C recommendation):
- Symptoms or signs of urethral discharge in men
- Vaginal discharge with risk factor for STI (age < 30 years, new sexual partner)
- Mucopurulent cervicitis
- Persons diagnosed with any other STI
- Sexual partner of persons with an STI or PID
- Acute epididymo-orchitis in a male aged < 40 years
- Acute pelvic inflammatory disease
- When screening young adults (< 25 years of age) for sexually transmitted infections
- When screening individuals with new or multiple recent sexual partners
- Purulent conjunctivitis in a neonate or adult
- Mother of a newborn with ophthalmia neonatorum
- Unplanned termination of pregnancy in places or populations of high gonorrhoea prevalence
- When intrauterine interventions are performed in areas of high gonorrhoea prevalence
The Management of Lymphogranuloma Venereum (de Vries et al., 2019): Lymphogranuloma venereum (LGV) is a condition caused by chlamydia. The clinical features can vary, depending on the site of inoculation (genital versus rectum) and can include hemorrhagic proctitis, lymphadenopathy, papule or pustule formation, and buboes. Reactive inflammatory responses or physical signs of in infection may include “constitutional symptoms such as low-grade fever, chills, malaise, myalgia, [and] arthralgia.” Regarding a diagnosis of lymphogranuloma venereum (LGV), “a sample tested C. trachomatis positive with a commercial nucleic acid amplification test (NAAT) platform should be confirmed with an LGV discriminatory NAAT.” Further, “For sensitive and specific detection of LGV genovar (L1, L2 and L3, including subvariant)-specific C. trachomatis DNA, laboratories are currently recommended to use a two-step procedure (1,B):
- “A commercially available NAAT is used to detect C. trachomatis DNA/RNA in suspected clinical samples. These tests cannot discriminate between LGV and non-LGV genovars. Although no commercially available C. trachomatis NAATs are FDA-cleared for extragenital specimens, for several NAATs sufficient evidence supports the use of these tests for the detection of C. trachomatis DNA/RNA also in rectal and pharyngeal C. trachomatis infections. Some C. trachomatis NAAT are CE-labelled for use on rectal and pharyngeal samples in Europe.
- If C. trachomatis DNA/RNA is detected, LGV genovar specific C. trachomatis DNA should be detected from the same specimen. There are multiplex NAATs for genital ulcerative disease that detect LGV but these have not yet been appropriately evaluated in the context of rectal LGV. Different in-house or laboratory-developed NAATs have been designed and used. The sensitivities of these NAATs are generally lower than the commercially available C. trachomatis screening NAAT (de Vries et al., 2019).”
The Management of Syphilis (Janier et al., 2014; Janier et al., 2020): The three stages (primary, secondary, and tertiary) can be overlapping. Primary syphilis begins with appearance of an ulcer (also known as a chancre), usually in the anogenital region with regional lymphadenopathy. “Any anogenital ulcer should be considered syphilitic unless proven otherwise.” The secondary stage is characterized by “multisystem involvement due to bacteriaemia, within the first year but may recur up into the second year after infection” and can include skin rash, generalized lymphadenopathy, arthritis, hepatitis, splenomegaly, and kidney dysfunction. Early neurosyphilis can occur in secondary syphilis and can include “meningitis, cranial nerve palsies, auricular and ophthalmic abnormalities (such as uveitis, retinitis, otitis and papillar oedema).” They list the following as conditions of tertiary syphilis:
- “Gummatous syphilis: nodules/plaques or ulcers (skin, mucosae, visceral)”
- “Late neurosyphilis encompasses meningitis, cranial nerve dysfunction, meningovascular syphilis (stroke, myelitis) and parenchymatous neurosyphilis (general paresis, tabes dorsalis)”
- “Cardiovascular syphilis: aortic regurgitation, stenosis of coronary ostia, aortic aneurysm (mainly thoracic)”
The following guidelines were given regarding laboratory testing for T. pallidum:
- “Direct detection methods provide definitive diagnosis of syphilis.
- Darkfield examination (DFE) of chancres and erosive cutaneous lesions was the old gold standard method for definitive diagnosis. It gives immediate results. However, the method is labor intensive, subjective, and can result in some false positive and (many) false negative results. Due to the availability of more sensitive and specific tests (specifically the PCR), it is not recommended for routine diagnosis anymore.
- Polymerase chain reaction (PCR) testing is the preferred method particularly but not exclusively for oral and other lesions where contamination with commensal treponemes is likely. It can be performed using tissues, cerebrospinal fluid (CSF) or blood (although insensitive in the latter). There is no internationally approved PCR assay for T. pallidum and accordingly, it is crucial to select a strictly validated and quality-assured method and always use it with appropriate quality controls.
- Immunohistochemistry using a polyclonal antibody against T. pallidum can be efficient to identify treponemes in skin, mucosal and tissue lesions, but it is not suitable for routine diagnosis.
- Hybridization in tissues is not used for routine diagnosis.
- Warthin-Starry (argentic) staining on tissues is very difficult to perform and of limited value in most cases.
- (Direct fluorescent antibody test is obsolete)
- For molecular epidemiological typing, PCR, PCR-restriction fragment length polymorphism (RFLP) and/or DNA-sequencing (e.g. multilocus sequence typing (MLST) or whole genome sequencing) can be performed on clinical specimens. However, due to the highly conserved genome of T. pallidum the discriminatory ability of typing methods is in general low (Janier et al., 2020)”
Primary Screening Test(s)
- “TT [TPHA, MHA-TP, TPPA or EIA/ELISA/CLIA] — A TT-based screening algorithm, using by preference an automatized EIA/ELISA/CLIA, is used in many large, well-resourced European laboratories and is particularly suitable for automated high-throughput screening of asymptomatic populations including blood/plasma donors. The algorithm identifies persons with previous successful treatment of syphilis as well as those with untreated syphilis. It is usually more sensitive in detecting very early syphilis compared to the use of a screening NTT. However, it can also result in a high number of false positive tests (i.e. very low positive predictive value) in low-prevalence populations.
- NTT [RPR or VDRL] — A NTT-based screening algorithm; preferably quantitative (i.e. to detect prozone phenomenon in infectious syphilis), is still recommended in some countries. In this algorithm, only active (infectious) syphilis is detected, however, it has a lower sensitivity compared to using a TT as primary screening test, and in particular very early syphilis can be missed.
- TT combined with a NTT — This algorithm is particularly useful in cases where the suspicion of very early syphilis is high (recent chancre, contacts of syphilis cases etc.), because in some patients NTT may become reactive before TT (Janier et al., 2020).”
Confirmatory test(s) if any screening test is positive
- “In the case a TT being used alone as a primary screening test, if positive, a confirmatory TT of a different type is of limited value in informing treatment, but a reflex quantitative NTT (reaching at least 1:8 to 1:16 dilution) should be performed in all cases on the same serum (1, B). Although a confirmatory TT may be important for counselling, notification and may have a psychological impact, it has limited impact on treatment.69 In patients with a positive TT, a negative NTT and no suspicion of very early syphilis (no chancre), both tests should be repeated after 1 month (1, D). However, CLIA and EIA used in many European settings have suboptimal specificity, resulting in a low positive predictive value in low prevalence population. 22,49,56 If such tests are used, additionally a reflex confirmatory test by TPHA or TPPA should be performed (1, C).
- In the case a NTT alone is used as a primary screening test, a positive test must be followed by a reflex TT on the same serum. If quantitative NTT was not initially done, the NTT should be repeated quantitatively (1, B).
- In the case both a TT and a NTT are used as primary screening tests such as (EIA/ELISA/CLIA/TPHA/TPPA plus VDRL/RPR), the NTT must be performed quantitatively (if not initially done) in case of positive or discrepant screening tests (1, B).
- The IgG-immunoblot for Treponema pallidum has no added major value to other TT. It is expensive and interpretation of undetermined immunoblot is elusive (1 to 4 bands).
The Management of Chlamydia Trachomatis Infections (Lanjouw et al., 2016): “Appropriate testing of symptomatic and asymptomatic sexually active individual is recommended to identify and treat the C. trachomatis infections.” With a Grade A recommendation, they recommend using NAATs that identify specific nucleic acid, either DNA or RNA) of C. trachomatis “due to their superior sensitivity, specificity, and speed.”
The following list contains the indications for laboratory testing as recommended by the IUSTI with a Grade C recommendation (Lanjouw et al., 2016):
Indications for laboratory testing (Level of evidence IV; Grade C recommendation)
- Risk factor(s) for C. trachomatis infection and/or other STI (age < 25 years, new sexual contact in the last year, more than one partner in the last year)
- Symptoms or signs of urethritis in men
- Cervical or vaginal discharge with risk factor for STI
- Acute epididymo-orchitis in a male aged < 40 years or with risk factors for STI
- Acute pelvic pain and/or symptoms or signs of PID
- Proctitis/proctocolitis according to risk
- Purulent conjunctivitis in a neonate or adult
- Atypical neonatal pneumonia
- Persons diagnosed with other STI
- Sexual contact of persons with an STI or PID
- Termination of pregnancy
- Any intrauterine interventions or manipulations
The Management of Genital Herpes (Patel et al., 2017): The principle change to the IUSTI guidelines in this recent version is that “HSV DNA detection rather than cell culture is now the gold standard for diagnosis.” With a grade C recommendation, “serological testing is not routinely recommended in asymptomatic patients.” They note that there are specific groups where it may be useful, including pregnant women, sexual partners of HSV-positive people, those with a history of recurrent or atypical genital disease, and those with first-episode genital herpes whose differentiation may aid in counseling and management (because seroconversion happens typically at 90 days post-infection).
Male Training Center for Family Planning & Reproductive Health (MTC), Office of Population Affairs, Department of Health and Human Services
In general, the MTC recommends at least annual testing for chlamydia, gonorrhea, syphilis, HIV/AIDS, and Hepatitis C for anyone in an at-risk population, including MSM. For syphilis, certain populations require testing at 3 – 6 month intervals, including those who exchange sex for drugs, commercial sex workers, and young MSM.
The MTC does not recommend screening for pharyngeal chlamydia infections. They do recommend follow-up test three months after initial positive chlamydia test. They recommend using a urine-based NAAT for chlamydia for at-risk male populations under the age of 25, which include MSM, patients at STI clinics, and military personnel (under the age of 30), and inmates entering jails or detention centers (under the age of 30). Men who have had receptive anal intercourse in the preceding year should have a NAAT performed on a rectal swab to check for rectal chlamydial infection.
The MTC recommends using NAAT for gonorrhea testing of at-risk male adolescents and adults, including MSM. “Males with gonorrhea infection should be re-screened for reinfection at 3 months.” Annual exams for MSM include screening for urethral infections, pharyngeal infections using NAAT for those “who have had receptive oral intercourse” during the preceding year, and rectal infections using NAAT of rectal swabs for those “who have had receptive anal intercourse” during the preceding year. “More frequent STD screening (i.e., at 3 – 6 month intervals) is indicated for MSM who have multiple or anonymous partners (Marcell & Health, 2014).”
Canadian Guidelines on Sexually Transmitted Infections
“For anal warts, no specific testing is recommended to verify the presence or type of HPV as this will not alter management. Anal Pap and/or HPV testing may be of value to identify precancerous anal intraepithelial neoplasia (AIN) in high-risk groups … Although no products are currently licensed for these [pharyngeal] specimens in Canada, validated NAATs can be used to detect oropharyngeal N. gonorrhoeae and C. trachomatis infections. Confirmation of positives with culture or a second NAAT should be performed.” NAAT can be performed on first-void urine samples from male patients or vaginal swabs or urine samples obtained from female patients. Since NAAT allows for the testing of antimicrobial susceptibility in gonorrheal infections, “depending on the clinical situation, consideration should be given to using both culture and NAAT, especially in symptomatic patients.” For oral lesions of suspected HSV, they recommend using NAAT or to obtain fluid for culture. “NAATs approach sensitivities and specificities of 100%, with rapid turn-around of results.” For syphilis, “NAATs can be used as a non-serological method for identifying T. pallidum in mucosa and skin involve. They are very sensitive and specific. When genital lesions characteristic of early syphilis are present, clear serous fluid may be collected for dark-field microscopy, enabling observation of morphology and movement of the spirochetes for the detection of T. pallidum (not reliable for oral or rectal lesions)” (Chernesky et al., 2017).
American Academy of Pediatrics (AAP)
Chlamydia: The AAP recommends annual screening for sexually active females 25 years old or younger. They also recommend annual urethral and rectal chlamydia screenings for sexually active MSM, but more frequent screening (every 3 – 6 months) for those who are in a higher risk category, such as multiple partners, sex-for-drugs, and so on. Anyone who has been exposed to chlamydia in the past 60 days should also be tested. “Consider screening sexually active males annually in settings with high prevalence rates, such as jails or juvenile corrections facilities, national job training programs, STD clinics, high school clinics, and adolescent clinics for patients who have a history of multiple partners.” Anyone who has tested positive for chlamydia should be retested three months after receiving treatment.
Gonorrhea: Similar to chlamydia, the AAP recommends annual screening for sexually active females under the age of 25. “Routinely screen sexually active adolescent and young adults MSM for pharyngeal, rectal, and urethral gonorrhea infection annually if engaging in receptive oral or anal intercourse or insertive intercourse, respectively.” Again, like chlamydial infections, those participating in higher risk activities should be tested every 3 – 6 months. Anyone who has been exposed to gonorrhea in the past 60 days should also be tested. Finally, the screening recommendations for other males are similar to the recommendations concerning chlamydial infections. Anyone who has tested positive for gonorrhea should be retested three months after receiving treatment.
Syphilis: “The routine screening of nonpregnant, heterosexual adolescents is not recommended. However, screening is recommended for all sexually active adolescent and young adults MSM annually or every 3 to 6 months if high risk and can be considered for youth whose behaviors put them at higher risk” (Murray et al., 2014).
National Institute for Health and Care Excellence (NICE)
NICE released their guidelines concerning cancer of the upper aerodigestive tract in 2016 (with updates in 2018 online). Recommendation 1.6.1: “Test all squamous cell carcinomas of the oropharynx using p16 immunohistochemistry. Regard the p16 test result as positive only if there is strong nuclear and cytoplasmic staining in more than 70% of tumour cells.” In Recommendation 1.6.2: “Consider high-risk HPV DNA or RNA in-situ hybridisation in all p16-positive cancers of the oropharynx to confirm HPV status.” In explaining their recommendations, NICE states, “HPV testing is currently recommended in cancer of the oropharynx because it has significant prognostic implication” (NCCC, 2018).
Canadian Paediatric Society (CPS)
The 2018 update to the CPS practice point titled “Congenital syphilis: No longer just of historical interest" included the following:
“Syphilis serology should routinely be performed at the first prenatal visit, followed by appropriate maternal counselling and therapy, if reactive. Rescreening should occur at 28 to 32 weeks’ gestation and at delivery in high-risk women, including women who originate from a country with a high prevalence of syphilis. Routine rescreening should also be considered in areas experiencing outbreaks of heterosexual syphilis. If syphilis serology was not performed during pregnancy, newborns should not be discharged from hospital until maternal serology has been drawn and follow-up of results has been arranged. If the cause is not known for a hydropic or stillbirth newborn, the mother should be screened for syphilis postpartum (Robinson & Canadian Paediatric Society, 2018).”
The CPS practice point sexually transmitted infections in adolescents: Maximizing opportunities for optimal care (Allen et al., 2019) included the following table concerning what screening tests should be used for each condition. These guidelines were updated in 2019, and reaffirmed in 2020 (Allen et al., 2019).
Table 1: What screening tests should be used use to detect sexually transmitted infections?
|
What screening tests should be used use to detect sexually transmitted infections? |
||
|
Infection |
Screening tests/samples |
Follow-up testing |
|
Chlamydia |
NAAT is the most sensitive and specific test. Can be performed on urine, urethral swabs, vaginal or cervical swabs*
|
Test-of-cure 3 to 4 weeks after treatment:
– Second-line or alternative treatment was used – Re-exposure risk is high – An adolescent is pregnant |
|
Syphilis |
Serology remains the usual diagnostic test unless the patient has lesions compatible with syphilis |
Follow-up testing depends on the nature of infection, as follows: Late latent infection: Repeat serology 12 and 24 months after treatment Neurosyphilis: Repeat 6, 12, and 24 months after treatment |
|
Gonorrhea |
NAAT can be used to detect gonorrhea from urine, and urethral, vaginal and cervical swabs in symptomatic and asymptomatic individuals*
|
Test-of-cure (culture 3 to 7 days post-treatment or NAAT 2 to 3 weeks later) if:
– Antimicrobial resistance is a concern – Compliance is uncertain – Re-exposure risk is high – An adolescent is pregnant – Previous treatment failure – Pharyngeal or rectal infection – Infection is disseminated – Signs, symptoms persist post-treatment |
*Discuss specimen selection to ensure that the NAAT is validated for the specimen to be collected and the patient being tested. For example, NAAT testing has not been validated for children ≤ 12 years of age and for medico-legal specimens.
British Association for Sexual Health and HIV (BASHH)
UK National Guideline for the Management of Lymphogranuloma Venereum (White et al., 2013): “Commercial molecular diagnostic techniques to detect C. trachomatis remain the primary test of choice, with referral of C. trachomatis-positive specimens for molecular tests to confirm the presence of LGV-associated DNA.” Testing should be performed on anyone exhibiting symptoms of an LGV infection, including hemorrhagic proctitis, primary lesions, suspected LGV-associated pharyngitis, secondary lesions, buboes, lymphadenitis, and/or lymphadenopathy. Main diagnostic techniques include using either NAATs, “culture on cycloheximide-treated McCoy cells of material from suspected LGV lesions,” or serology testing. “Serology cannot necessarily distinguish past from current LGV infection, which might prove restrictive given the high number of recurrent LGV infections now seen in MSM.”
UK National Guideline for the Management of Anogenital Herpes (Patel et al., 2015): The clinical signs and symptoms of an HSV infection can include “painful ulceration, dysuria, vaginal or urethral discharge” as well as systemic symptoms of fever and myalgia. Other signs can include bilateral lymphadenitis — although, alternating sides can occur in subsequent episodes — and proctitis. With a Grade C recommendation, “The confirmation and typing of the infection and its type, by direct detection of HSV in genital lesions, are essential for diagnosis, prognosis, counselling, and management.” BASHH gives an “A” recommendation of directly testing swabs from either anogenital lesions or the rectal mucosa in suspected proctitis. They recommend with a “B” rating that virus typing be performed to differentiate HSV-1 from HSV-2 in newly diagnosed cases of genital herpes. NAATs are the preferred testing method (grade “A” recommendation) since HSV culture tests can miss around 30% of PCR-positive samples.
UK National Guideline for the Management of Infection with Chlamydia Trachomatis (updated 2018) (Nwokolo et al., 2016): “Testing for genital and extra-genital chlamydia should be performed using NAATs (Grade B).” MSM who test positive for both HIV and chlamydia should be tested for LGV even if asymptomatic for the latter (Grade B). They give a Grade B recommendation for LGV testing in patients presenting with proctitis and a Grade C recommendation for treating both sexes presenting with proctitis the same.
The guidelines were updated in 2018, but NAAT testing is still considered the current standard of care for all chlamydia cases by the BASHH; “Although no test is 100% sensitive or specific, NAATs are known to be more sensitive and specific than EIAs” (BASHH, 2018).
UK National Guidelines on the Management of Syphilis (updated 2017, 2019) (Kingston et al., 2016): They recommend (2A) “where appropriate expertise and equipment are available, perform dark ground microscopy on possible chancres” and (1A) that “T. pallidum testing by PCR is appropriate on lesions where the organism may be expected to be located.” Within the section on serology, they recommend (1B) that “An EIA/CLIA, preferably detecting both IgM and IgG is the screening test of choice”; “positive screening tests should be confirmed with a different treponemal test (not the FTA-abs) and a second specimen for confirmatory testing obtained” (1B); “a quantitative RPR or VDRL should be performed when screening tests are positive” (1A); and (1B) repeat testing for syphilis at 6 and 12 weeks if an isolated episode and “at two weeks after possible chancres that are dark-ground and/or PCR negative are observed.” These guidelines were updated in 2017 and 2019, but diagnostic testing methods were not changed.
Cumulative Guideline Table
|
Year & Society |
Condition |
Microorganism |
Recommendation |
|
2022 NCCN |
Anal Carcinoma |
HPV |
HPV linked to anal cancers and HPV positivity linked to positive OS |
|
2021 NCCN |
Cervical Cancer |
HPV |
Overwhelming evidence of link between HPV and cervical cancer; chronic HPV infection status used in aiding treatment/surgical options |
|
2022 NCCN |
Head and Neck Cancers/ Oropharyngeal Cancer |
HPV |
Requires HPV p16 testing by IHC; HPV status is imperative in determining therapy |
|
2021 NCCN |
Occult Primary Cancers (Squamous Cell Carcinoma) |
HPV |
If clinical presentation in the head and neck nodes is noted, check p16 IHC and ISH results |
|
2022 NCCN |
Penile Cancer |
HPV |
HPV linked to penile cancer; HPV status of lesions important for determining therapy |
|
2021 NCCN |
Vulvar Cancer (Squamous Cell Carcinoma) |
HPV |
HPV linked to vulvar cancer, especially HSIL; recommends HPV testing for “appropriate patients” |
|
2021 USPSTF |
NA |
Chlamydia, Gonorrhea |
Testing in sexually active women age 24 or younger and older women of at-risk populations; insufficient evidence concerning routinely screening in general population of males |
|
2014 USPSTF |
Oropharyngeal Cancer |
HPV |
Insufficient evidence to assess testing for HPV in cases of asymptomatic oropharyngeal cancer |
|
2016 USPSTF |
Asymptomatic Genital Herpes |
HSV-2 |
Do not recommend testing asymptomatic patients for HSV-2 |
|
2016 USPSTF |
NA |
Syphilis |
Grade A recommendation for screening asymptomatic patients of HIGH RISK categories but they do NOT recommend screening in asymptomatic patients not in high risk categories; recommend screening HIV-positive men and MSM every three months |
|
2021 CDC |
Genital, Anal, or Perianal Ulcers |
Syphilis, HSV |
Recommends syphilis serology, darkfield exam, or PCR testing if possible; culture or PCR for genital herpes; serologic testing for type-specific HSV antibody |
|
2021 CDC |
NA |
Syphilis |
Darkfield examination of exudate can be used for early diagnosis; presumptive diagnosis requires use of two tests—both a treponemal test and a non-treponemal test; any signs of CNS infection require additional testing |
|
2021 CDC |
NA |
Chlamydia |
Testing of women under age of 25 as well as older women and men if they fall in a high-risk category; do NOT recommend testing of asymptomatic men and older women |
|
2021 CDC |
NA |
Gonorrhea |
Testing of women under age of 25 as well as older women and men if they fall in a high-risk category; do NOT recommend testing of asymptomatic men and older women; men showing signs of urethral gonococcal infection should be tested |
|
2021 CDC |
NA |
HPV |
Recommends against using oncogenic HPV testing for asymptomatic men, women aged 25 and over, or for general STI testing.
There is no approved test for HPV in men, and routine testing is not recommended for anal, penile, or throat cancers in men. |
|
2021 CDC |
Anogenital Warts |
HPV |
“HPV testing is not recommended for anogenital wart diagnosis, because test results are not confirmatory and do not guide genital wart management.” |
|
2021 CDC |
Cervical Screening |
HPV |
For women aged 30 or older, HPV testing can be part of cervical screening. For women ages 30 – 65, if co-testing Pap test and HR-HPV, then frequency is every 5 years … if only doing a Pap test, the frequency is every 3 years
HPV tests to screen for cervical cancer are recommended for women 30 years and older. They are not recommended to screen, men, adolescents, or women under the age of 30. |
|
2019 IUSTI |
Anogenital Warts |
HPV |
Do not recommend HPV testing for symptomatic anogenital warts since it adds no information for clinical use. |
|
2020 IUSTI |
NA |
Gonorrhea |
Culture testing is only method to determine antimicrobial susceptibility, but NAAT testing is more sensitive. Includes list of symptoms for testing. |
|
2019 IUSTI |
Lymphogranuloma venereum |
Chlamydia |
To diagnose LGV, a sample tested C. trachomatis positive with a commercial nucleic acid amplification test (NAAT) platform should be confirmed with an LGV discriminatory NAAT. For sensitive and specific LGV detection, laboratories are recommended to use a two-step procedure. |
|
2014, 2020 IUSTI |
NA |
Syphilis |
Like the CDC, they recommend a two-test method for diagnosing syphilis (one non-treponema test and one treponema test) if any initial screening test is positive |
|
2015 IUSTI (published in 2016) |
NA |
Chlamydia |
Recommends using an NAAT for chlamydia testing and lists signs/symptoms that require testing |
|
2017 IUSTI |
Genital herpes |
HSV |
Typically, does not recommend testing in asymptomatic patients; HSV DNA detection now replaces culture as gold standard |
|
2014 MTC |
NA |
Chlamydia |
Do not recommend pharyngeal screenings. Do recommend NAAT of at-risk groups with a 3-month follow-up test for patients who tested positive |
|
2014 MTC |
NA |
Gonorrhea |
Do recommend annual NAAT of at-risk groups with a 3-month follow-up test for patients who tested positive; more frequent testing in certain MSM populations |
|
2014 MTC |
NA |
Syphilis |
Do recommend annual testing of at-risk groups with 3-6 month testing of certain populations (commercial sex workers, inmates of correctional facilities, persons who exchange sex for drugs, and so on) |
|
2017 Canadian Guidelines on STIs |
NA |
Chlamydia, Syphilis, Gonorrhea, HSV, and HPV |
NAATs are more specific and sensitive than culture testing when available. For gonorrheal infections, only culture can test for antimicrobial susceptibility in gonorrhea. |
|
2014 AAP |
Adolescents & young adults |
Chlamydia, Gonorrhea |
All sexually active young women (under the age of 25) and MSM should have annual screenings. For those at higher risk, they should be screened every 3-6 months. Anyone who tests positive should be retested 3 months after receiving treatment. |
|
2014 AAP |
Adolescents & young adults |
Syphilis |
Do NOT recommend routine screening except for sexually active young MSM. |
|
2016 NICE |
Oropharyngeal Cancers |
HPV |
Test all carcinomas of the oropharynx using p16 IHC; consider using high-risk HPV DNA/RNA in situ hybridization in all p16-positive cancers |
|
2018 CPS |
Pregnant women |
Syphilis |
Testing at first prenatal visit as well as 28-32 weeks; if not tested during pregnancy, child does not leave the hospital without being tested |
|
2020 CPS |
Adolescents/young adults |
Chlamydia, Syphilis, Gonorrhea |
See detailed testing and frequency in table within the guidelines above |
|
2015 BASHH (published in 2016) |
NA |
Syphilis |
Dark-field microscopy or PCR tests can be performed. For serology, EIA/CLIA is the screening test of choice (preferably where both IgM and IgG are detected). Positive tests must be followed by a quantitative RPR or VDRL. |
|
2013 BASHH |
Suspected LGV |
Chlamydia |
Testing should use either NAAT, culture testing, or serology; however, the latter cannot distinguish current from past infections. |
|
2014 BASHH (published in 2015) |
Anogenital herpes |
HSV |
NAAT is preferred over other forms of testing (“A” grade). Differentiation of virus type should be determined on new cases of genital herpes (“B” grade). |
|
2015, 2018 BASHH |
NA |
Chlamydia |
Test for chlamydia using NAATs. Both sexes presenting with proctitis should be treated the same with respect to LGV testing. HIV-positive men with chlamydia should also be tested for LGV, even if asymptomatic. |
|
Abbreviations: CLIA = chemiluminescent assay; EIA = enzyme immunoassay; GC = gonococcal; HPV = human papillomavirus; HR-HPV = high risk or oncogenic HPV testing; HSIL = high-grade squamous intraepithelial lesions; HSV = herpes simplex virus; IHC = immunohistochemistry; LGV = lymphogranuloma venereum; MSM = men having sex with men; NA = not applicable; NAAT = nucleic acid amplification testing; OS = overall survival; RPR = rapid plasma reagin test; VDRL = Venereal Diseases Research Laboratory carbon antigen test |
|||
References
- Albrecht, M. A. (2020, 12/22/2020). Epidemiology, clinical manifestations, and diagnosis of genital herpes simplex virus infection. Retrieved 06/30/2022 from https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-genital-herpes-simplex-virus-infection
- Allen, U. D., MacDonald, N. E., & Top, K. (2019). Diagnosis and management of sexually transmitted infections in adolescents. https://www.cps.ca/en/documents/position/sexually-transmitted-infections
- Arbyn, M., Roelens, J., Simoens, C., Buntinx, F., Paraskevaidis, E., Martin-Hirsch, P. P., & Prendiville, W. J. (2013). Human papillomavirus testing versus repeat cytology for triage of minor cytological cervical lesions. Cochrane Database Syst Rev(3), Cd008054. https://doi.org/10.1002/14651858.CD008054.pub2
- BASHH. (2018, 09/26/2018). BASHH CLINICAL EFFECTIVENESS GROUP Update on the treatment of Chlamydia trachomatis (CT) infection. https://www.bashhguidelines.org/current-guidelines/urethritis-and-cervicitis/chlamydia-2015/
- BD. (2020). BD receives FDA Approval for HPV Test with Extended Genotyping Capabilities. https://www.bd.com/en-us/company/news-and-media/press-releases/july-22-2020-bd-receives-fda-approval-for-hpv-test-with-extended-genotyping-capabilities
- Brischetto, A., Gassiep, I., Whiley, D., & Norton, R. (2018). Retrospective Review of Treponema pallidum PCR and Serology Results: Are Both Tests Necessary? J Clin Microbiol, 56(5). https://doi.org/10.1128/jcm.01782-17
- Bristow, C. C., Morris, S. R., Little, S. J., Mehta, S. R., & Klausner, J. D. (2019). Meta-analysis of the Cepheid Xpert(®) CT/NG assay for extragenital detection of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections. Sex Health, 16(4), 314-319. https://doi.org/10.1071/sh18079
- Cantor, A. G., Pappas, M., Daeges, M., & Nelson, H. D. (2016). Screening for syphilis: Updated evidence report and systematic review for the us preventive services task force. JAMA, 315(21), 2328-2337. https://doi.org/10.1001/jama.2016.4114
- Castle, P. E., Stoler, M. H., Wright, T. C., Jr., Sharma, A., Wright, T. L., & Behrens, C. M. (2011). Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol, 12(9), 880-890. https://doi.org/10.1016/s1470-2045(11)70188-7
- CDC. (2017, 09/26/2017). The State of STDs - Infographic. Centers for Disease Control and Prevention. Retrieved 07/20/2018 from https://www.cdc.gov/std/stats16/infographic.htm
- CDC. (2021a, 07/22/2021). Chlamydia - CDC Fact Sheet (Detailed). Centers for Disease Control and Prevention. Retrieved 07/28/2021 from https://www.cdc.gov/std/chlamydia/stdfact-chlamydia-detailed.htm
- CDC. (2021b, 07/22/2021). Genital Herpes - CDC Fact Sheet (Detailed). Centers for Disease Control and Prevention. Retrieved 07/28/2021 from https://www.cdc.gov/std/herpes/stdfact-herpes-detailed.htm
- CDC. (2021c, 01/19/2021). Genital HPV Infection - Fact Sheet. Centers for Disease Control and Prevention. Retrieved 07/28/2021 from https://www.cdc.gov/std/hpv/stdfact-hpv.htm
- CDC. (2021d, 07/22/2021). Gonorrhea - CDC Fact Sheet (Detailed Version). Centers for Disease Control and Prevention. Retrieved 07/28/2021 from https://www.cdc.gov/std/gonorrhea/stdfact-gonorrhea-detailed.htm
- CDC. (2021e). Pre-Exposure Prophylaxis (PrEP). Centers for Disease Control and Prevention. https://www.cdc.gov/hiv/clinicians/prevention/prep.html
- CDC. (2021f). Sexually Transmitted Infections Treatment Guidelines,2021. Retrieved 07/28/2021 from https://www.cdc.gov/std/treatment-guidelines/STI-Guidelines-2021.pdf
- CDC. (2021g, 04/22/2021). Syphilis-CDC Fact Sheet (Detailed). Centers for Disease Control and Prevention. Retrieved 07/28/2021 from https://www.cdc.gov/std/syphilis/stdfact-syphilis-detailed.htm
- CDC. (2021h, 07/22/2021). Trichomoniasis. Retrieved 07/28/2021 from https://www.cdc.gov/std/trichomonas/default.htm
- CDC. (2021i, 07/22/2021). Trichomoniasis - CDC Fact Sheet. Retrieved 07/28/2021 from https://www.cdc.gov/std/trichomonas/stdfact-trichomoniasis.htm
- CDC. (2022a, 04/18/2022). HPV & Men Fact Sheet. https://www.cdc.gov/std/hpv/stdfact-hpv-and-men.htm
- CDC. (2022b). Sexually Transmitted Infections Treatment Guidelines, 2021 - Adolescents. https://www.cdc.gov/std/treatment-guidelines/adolescents.htm
- Cepheid. (2022a). Xpert CT/NG Datasheet. https://cepheid.widen.net/s/24ygfdtuxc
- Cepheid. (2022b). Xpert® CT/NG. https://www.cepheid.com/Package%20Insert%20Files/Xpert-CTNG-US-ENGLISH-Package-Insert-301-0234--Rev-K.pdf
- Chernesky, M., Fisher, W. A., Gale-Rowe, M., Labbé, A., Lau, T. T. Y., Lee, E., Martin, I., Ogilvie, G., Read, R., Robinson, J., Romanowski, B., Ryan, B., Singh, A., Steben, M., Wong, T., & Yudin, M. H. (2017, 04/20/2017). Canadian Guidelines on Sexually Transmitted Infections-Laboratory diagnosis of sexually transmitted infections. Public Health Agency of Canada. https://ipac-canada.org/photos/custom/Members/pdf/Laboratory%20Diagnosis%20of%20STI_April%202017_final-5.pdf
- Cook, R. L., Hutchison, S. L., Ostergaard, L., Braithwaite, R. S., & Ness, R. B. (2005). Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med, 142(11), 914-925.
- Cosentino, L. A., Danby, C. S., Rabe, L. K., Macio, I., Meyn, L. A., Wiesenfeld, H. C., & Hillier, S. L. (2017). Use of Nucleic Acid Amplification Testing for Diagnosis of Extragenital Sexually Transmitted Infections. J Clin Microbiol, 55(9), 2801-2807. https://doi.org/10.1128/jcm.00616-17
- Davidson, K. W., Barry, M. J., Mangione, C. M., Cabana, M., Caughey, A. B., Davis, E. M., Donahue, K. E., Doubeni, C. A., Krist, A. H., Kubik, M., Li, L., Ogedegbe, G., Pbert, L., Silverstein, M., Simon, M. A., Stevermer, J., Tseng, C. W., & Wong, J. B. (2021). Screening for Chlamydia and Gonorrhea: US Preventive Services Task Force Recommendation Statement. JAMA, 326(10), 949-956. https://doi.org/10.1001/jama.2021.14081
- de Vries, H. J. C., de Barbeyrac, B., de Vrieze, N. H. N., Viset, J. D., White, J. A., Vall-Mayans, M., & Unemo, M. (2019). 2019 European guideline on the management of lymphogranuloma venereum. J Eur Acad Dermatol Venereol, 33(10), 1821-1828. https://doi.org/10.1111/jdv.15729
- FDA. (2012a, 12/27/2012). 501(k) Premarket Notification Xpert CT/NG. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K121710
- FDA. (2012b, 12/27/2012). 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY K121710. https://www.accessdata.fda.gov/cdrh_docs/reviews/K121710.pdf
- FDA. (2019a, 05/23/2019). 501(k) Premarket Notification Xpert CT/NG, GeneXpert Dx System, GeneXpert Infinity-48s and GeneXpert Infinity-80 Systems, GeneXpert Infinity-48 System, Xpert Vaginal/Endocervical Specimen Collection, Xpert Urine Specimen Collection Kit, Xpert Swab Specimen Collection Kit. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K190441
- FDA. (2019b, 05/23/2019). 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY K190441. https://www.accessdata.fda.gov/cdrh_docs/reviews/K190441.pdf
- FDA. (2021, 07/26/2021). BD ONCLARITY HPV ASSAY. U.S. Food & Drug Administration. https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pma&id=391601
- Feldman, S., & Crum, C. P. (2022, 05/02/2022). Cervical cancer screening tests: Techniques for cervical cytology and human papillomavirus testing. Retrieved 06/30/2022 from https://www.uptodate.com/contents/cervical-cancer-screening-tests-techniques-for-cervical-cytology-and-human-papillomavirus-testing
- Feltner, C., Grodensky, C., Ebel, C., & et al. (2016). Serologic screening for genital herpes: An updated evidence report and systematic review for the us preventive services task force. JAMA, 316(23), 2531-2543. https://doi.org/10.1001/jama.2016.17138
- Gaydos, C. A., Ako, M. C., Lewis, M., Hsieh, Y. H., Rothman, R. E., & Dugas, A. F. (2019). Use of a Rapid Diagnostic for Chlamydia trachomatis and Neisseria gonorrhoeae for Women in the Emergency Department Can Improve Clinical Management: Report of a Randomized Clinical Trial. Ann Emerg Med, 74(1), 36-44. https://doi.org/10.1016/j.annemergmed.2018.09.012
- Ghanem, K. G. (2022, 05/26/2022). Clinical manifestations and diagnosis of Neisseria gonorrhoeae infection in adults and adolescents. Retrieved 06/30/2022 from https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-neisseria-gonorrhoeae-infection-in-adults-and-adolescents
- Ghanem, K. G., & Tuddenham, S. (2022, 05/26/2022). Screening for sexually transmitted infections. Wolters Kluwer. Retrieved 06/30/2022 from https://www.uptodate.com/contents/screening-for-sexually-transmitted-infections
- Gilson, R., Nugent, D., Werner, R. N., Ballesteros, J., & Ross, J. (2020). 2019 IUSTI-Europe guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol, 34(8), 1644-1653. https://doi.org/10.1111/jdv.16522
- Glass, N., Nelson, Heidi D. (2021). Screening for Genital Herpes Simplex: A Brief Update for the U.S. Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Home/GetFile/1/733/herpesup/pdf
- Golden, M., O'Donnell, M., Lukehart, S., Swenson, P., Hovey, P., Godornes, C., Romano, S., & Getman, D. (2019). Treponema pallidum Nucleic Acid Amplification Testing To Augment Syphilis Screening among Men Who Have Sex with Men. J Clin Microbiol, 57(8). https://doi.org/10.1128/jcm.00572-19
- Guenat, D., Launay, S., Riethmuller, D., Mougin, C., & Pretet, J. L. (2016). Validation of Novaprep((R)) HQ+ liquid-based cytology medium for high-risk human papillomavirus detection by hc2. Infect Agent Cancer, 11, 41. https://doi.org/10.1186/s13027-016-0092-7
- Guy, R. J., Causer, L. M., Klausner, J. D., Unemo, M., Toskin, I., Azzini, A. M., & Peeling, R. W. (2017). Performance and operational characteristics of point-of-care tests for the diagnosis of urogenital gonococcal infections. Sex Transm Infect, 93(S4), S16-s21. https://doi.org/10.1136/sextrans-2017-053192
- Hicks, C. B., & Clement, M. (2021a, 11/05/2020). Syphilis: Epidemiology, pathophysiology, and clinical manifestations in HIV-uninfected patients. Retrieved 07/28/2021 from https://www.uptodate.com/contents/syphilis-epidemiology-pathophysiology-and-clinical-manifestations-in-hiv-uninfected-patients
- Hicks, C. B., & Clement, M. (2021b, 07/06/2021). Syphilis: Screening and diagnostic testing. Retrieved 07/28/2021 from https://www.uptodate.com/contents/syphilis-screening-and-diagnostic-testing
- Hsu, K. (2022, 04/08/2022). Clinical manifestations and diagnosis of Chlamydia trachomatis infections. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-chlamydia-trachomatis-infections
- Janier, M., Hegyi, V., Dupin, N., Unemo, M., Tiplica, G. S., Potocnik, M., French, P., & Patel, R. (2014). 2014 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol, 28(12), 1581-1593. https://doi.org/10.1111/jdv.12734
- Janier, M., Unemo, M., Dupin, N., Tiplica, G. S., Potocnik, M., & Patel, R. (2020). 2020 European guideline on the management of syphilis. Acta Clin Belg. https://doi.org/10.1080/17843286.2020.1773112
- Juarez-Figueroa, L., Uribe-Salas, F., Garcia-Cisneros, S., Olamendi-Portugal, M., & Conde-Glez, C. J. (2007). Evaluation of a rapid strip and a particle agglutination tests for syphilis diagnosis. Diagn Microbiol Infect Dis, 59(2), 123-126. https://doi.org/10.1016/j.diagmicrobio.2007.04.008
- Kelly, H., Coltart, C. E. M., Pant Pai, N., Klausner, J. D., Unemo, M., Toskin, I., & Peeling, R. W. (2017). Systematic reviews of point-of-care tests for the diagnosis of urogenital Chlamydia trachomatis infections. Sex Transm Infect, 93(S4), S22-s30. https://doi.org/10.1136/sextrans-2016-053067
- Kingston, M., French, P., Higgins, S., McQuillan, O., Sukthankar, A., Stott, C., McBrien, B., Tipple, C., Turner, A., Sullivan, A. K., Radcliffe, K., Cousins, D., FitzGerald, M., Fisher, M., Grover, D., Higgins, S., Kingston, M., Rayment, M., & Sullivan, A. (2016). UK national guidelines on the management of syphilis 2015. Int J STD AIDS, 27(6), 421-446. https://doi.org/10.1177/0956462415624059
- Lanjouw, E., Ouburg, S., de Vries, H. J., Stary, A., Radcliffe, K., & Unemo, M. (2016). 2015 European guideline on the management of Chlamydia trachomatis infections. Int J STD AIDS, 27(5), 333-348. https://doi.org/10.1177/0956462415618837
- LeFevre, M. L. (2014). Screening for Chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med, 161(12), 902-910. https://doi.org/10.7326/m14-1981
- Liu, T. Y., Xie, R., Luo, L., Reilly, K. H., He, C., Lin, Y. Z., Chen, G., Zheng, X. W., Zhang, L. L., & Wang, H. B. (2014). Diagnostic validity of human papillomavirus E6/E7 mRNA test in cervical cytological samples. J Virol Methods, 196, 120-125. https://doi.org/10.1016/j.jviromet.2013.10.032
- Marcell, A. V., & Health, M. T. C. f. F. P. a. R. (2014). Preventive Male Sexual and Reproductive Health Care: Recommendations for Clinical Practice. U.S. Department of Health and Human Services. Retrieved 07/12/2018 from http://content.guidelinecentral.com/guideline/get/pdf/2787
- Moyer, V. A. (2014). Screening for oral cancer: U.S. preventive services task force recommendation statement. Ann Intern Med, 160(1), 55-60. https://doi.org/10.7326/M13-2568
- Murray, P., Braverman, P., Adelman, W., Breuner, C., Levine, D., Marcell, A. V., PJ, M., O'Brien, R., & Burstein, G. (2014). Screening for nonviral sexually transmitted infections in adolescents and young adults. Pediatrics, 134(1), e302-311. https://doi.org/10.1542/peds.2014-1024
- NCCC. (2018). National Institute for Health and Care Excellence: Clinical Guidelines. In Cancer of the Upper Aerodigestive Tract: Assessment and Management in People Aged 16 and Over. National Institute for Health and Care Excellence (UK) Copyright (c) National Collaborating Centre for Cancer. https://www.nice.org.uk/guidance/ng36/evidence/full-guideline-2307980269
- NCCN. (2021a, 10/26/2021). NCCN Clinical Practice Guidelines in Oncology Cervical Cancer Version 1.2022. Retrieved 06/30/2022 from https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
- NCCN. (2021b, 10/07/2021). NCCN Clinical Practice Guidelines in Oncology Vulvar Cancer (Squamous Cell Carcinoma) Version 1.2022. Retrieved 06/30/2022 from https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf
- NCCN. (2021c, 09/02/2021). NCCN Clinical Practice Guidelines Occult Primary (Cancer of Unknown Primary [CUP]). Retrieved 06/30/2022 from https://www.nccn.org/professionals/physician_gls/pdf/occult.pdf
- NCCN. (2022a, 03/02/2022). NCCN Clinical Practice Guidelines in Oncology Anal Carcinoma Version 1.2022. Retrieved 06/30/2022 from https://www.nccn.org/professionals/physician_gls/pdf/anal.pdf
- NCCN. (2022b, 04/26/2022). NCCN Clinical Practice Guidelines in Oncology Head and Neck Cancers Version 2.2022. Retrieved 06/30/2022 from https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- NCCN. (2022c, 01/26/2022). NCCN Clinical Practice Guidelines in Oncology Penile Cancer Version 2.2022. Retrieved 06/30/2022 from https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf
- Nwokolo, N. C., Dragovic, B., Patel, S., Tong, C. Y., Barker, G., & Radcliffe, K. (2016). 2015 UK national guideline for the management of infection with Chlamydia trachomatis. Int J STD AIDS, 27(4), 251-267. https://doi.org/10.1177/0956462415615443
- Palefsky, J. M. (2022, 06/17/2022). Human papillomavirus infections: Epidemiology and disease associations. https://www.uptodate.com/contents/human-papillomavirus-infections-epidemiology-and-disease-associations
- Papp, J. R., Schachter, J., Gaydos, C. A., & Van Der Pol, B. (2014). Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep, 63(Rr-02), 1-19. https://www.cdc.gov/mmwr/pdf/rr/rr6302.pdf
- Patel, R., Green, J., Clarke, E., Seneviratne, K., Abbt, N., Evans, C., Bickford, J., Nicholson, M., O'Farrell, N., Barton, S., FitzGerald, M., & Foley, E. (2015). 2014 UK national guideline for the management of anogenital herpes. Int J STD AIDS, 26(11), 763-776. https://doi.org/10.1177/0956462415580512
- Patel, R., Kennedy, O. J., Clarke, E., Geretti, A., Nilsen, A., Lautenschlager, S., Green, J., Donders, G., van der Meijden, W., Gomberg, M., Moi, H., & Foley, E. (2017). 2017 European guidelines for the management of genital herpes. Int J STD AIDS, 28(14), 1366-1379. https://doi.org/10.1177/0956462417727194
- Pham, M. D., Wise, A., Garcia, M. L., Van, H., Zheng, S., Mohamed, Y., Han, Y., Wei, W. H., Yin, Y. P., Chen, X. S., Dimech, W., Braniff, S., Technau, K. G., Luchters, S., & Anderson, D. A. (2020). Improving the coverage and accuracy of syphilis testing: The development of a novel rapid, point-of-care test for confirmatory testing of active syphilis infection and its early evaluation in China and South Africa. EClinicalMedicine, 24, 100440. https://doi.org/10.1016/j.eclinm.2020.100440
- Riley, L. E., & Wald, A. (2022, 02/10/2022). Genital herpes simplex virus infection and pregnancy. https://www.uptodate.com/contents/genital-herpes-simplex-virus-infection-and-pregnancy
- Robinson, J., & Canadian Paediatric Society, I. D. a. I. C. (2018, 04/06/2018). Congenital syphilis: No longer just of historical interest. Canadian Paediatric Society. Retrieved 07/16/2018 from https://www.cps.ca/en/documents/position/congenital-syphilis
- Tsang, R. S., Martin, I. E., Lau, A., & Sawatzky, P. (2007). Serological diagnosis of syphilis: comparison of the Trep-Chek IgG enzyme immunoassay with other screening and confirmatory tests. FEMS Immunol Med Microbiol, 51(1), 118-124. https://doi.org/10.1111/j.1574-695X.2007.00289.x
- Tshomo, U., Franceschi, S., Tshokey, T., Tobgay, T., Baussano, I., Tenet, V., Snijders, P. J., Gheit, T., Tommasino, M., Vorsters, A., & Clifford, G. M. (2017). Evaluation of the performance of Human Papillomavirus testing in paired urine and clinician-collected cervical samples among women aged over 30 years in Bhutan. Virol J, 14(1), 74. https://doi.org/10.1186/s12985-017-0744-2
- Unemo, M. (2020). 2020 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. https://iusti.org/wp-content/uploads/2020/10/IUSTI-Gonorrhoea-2020.pdf
- USPSTF. (2019). Preexposure Prophylaxis for the Prevention of HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA, 321(22), 2203-2213. https://doi.org/10.1001/jama.2019.6390
- White, J., O'Farrell, N., & Daniels, D. (2013). 2013 UK National Guideline for the management of lymphogranuloma venereum: Clinical Effectiveness Group of the British Association for Sexual Health and HIV (CEG/BASHH) Guideline development group. Int J STD AIDS, 24(8), 593-601. https://doi.org/10.1177/0956462413482811
- Wong, E. H., Klausner, J. D., Caguin-Grygiel, G., Madayag, C., Barber, K. O., Qiu, J. S., Liska, S., & Pandori, M. W. (2011). Evaluation of an IgM/IgG sensitive enzyme immunoassay and the utility of index values for the screening of syphilis infection in a high-risk population. Sex Transm Dis, 38(6), 528-532. https://doi.org/10.1097/OLQ.0b013e318205491a
- Workowski, K. A., & Bolan, G. A. (2015). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep, 64(Rr-03), 1-137. http://dx.doi.org/
- Yao, Y. L., Tian, Q. F., Cheng, B., Cheng, Y. F., Ye, J., & Lu, W. G. (2017). Human papillomavirus (HPV) E6/E7 mRNA detection in cervical exfoliated cells: a potential triage for HPV-positive women. J Zhejiang Univ Sci B, 18(3), 256-262. https://doi.org/10.1631/jzus.B1600288
- Zhiyan, L., Meiling, W., Ping, L., Jinhua, D., Zhenlin, Y., & Zhenru, F. (2015). Consistency Between Treponema pallidum Particle Agglutination Assay and Architect Chemiluminescent Microparticle Immunoassay and Characterization of Inconsistent Samples. J Clin Lab Anal, 29(4), 281-284. https://doi.org/10.1002/jcla.21765
Coding Section
|
Code |
Number |
Description |
|
CPT |
82565 |
Creatinine; blood |
|
|
82575 |
Creatinine; clearance |
|
|
84702 |
Gonadotropin, chorionic (hCG); quantitative |
|
|
84703 |
Gonadotropin, chorionic (hCG); qualitative |
|
|
86592 |
Syphilis test, non-treponemal antibody; qualitative (e.g., VDRL, RPR, ART) |
|
|
86593 |
Syphilis test, non-treponemal antibody; quantitative |
|
|
86631 |
Antibody; Chlamydia |
|
|
86632 |
Antibody; Chlamydia, IgM |
|
|
86694 |
Antibody; herpes simplex, non-specific type test |
|
|
86695 |
Antibody; herpes simplex, type 1 |
|
|
86696 |
Antibody; herpes simplex, type 2 |
|
|
86701 |
Antibody; HIV-1 |
|
|
86702 |
Antibody; HIV-2 |
|
|
86703 |
Antibody; HIV-1 and HIV-2, single result |
|
|
86704 |
Hepatitis B core antibody (HBcAb); total |
|
|
86705 |
Hepatitis B core antibody (HBcAb); IgM antibody |
|
|
86706 |
Hepatitis B surface antibody (HBsAb) |
|
|
86780 |
Antibody; Treponema pallidum |
|
|
86803 |
Hepatitis C antibody |
|
|
86804 |
Hepatitis C antibody; confirmatory test (e.g., immunoblot) |
|
|
87081 |
Culture, presumptive, pathogenic organisms, screening only; |
|
|
87110 |
Culture, chlamydia, any source |
|
|
87181 |
Susceptibility studies, antimicrobial agent; agar dilution method, per agent (e.g., antibiotic gradient strip) |
|
|
87340 |
Infectious agent antigen detection by immunoassay technique, (e.g., enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], fluorescence immunoassay [FIA], immunochemiluminometric assay [IMCA]) qualitative or semiquantitative; hepatitis B surface antigen (HBsAg) |
|
|
87490 |
Infectious agent detection by nucleic acid (DNA or RNA); Chlamydia trachomatis, direct probe technique |
|
|
87491 |
Infectious agent detection by nucleic acid (DNA or RNA); Chlamydia trachomatis, amplified probe technique |
|
|
87492 |
Infectious agent detection by nucleic acid (DNA or RNA); Chlamydia trachomatis, quantification |
|
|
87528 |
Infectious agent detection by nucleic acid (DNA or RNA); Herpes simplex virus, direct probe technique |
|
|
87529 |
Infectious agent detection by nucleic acid (DNA or RNA); Herpes simplex virus, amplified probe technique |
|
|
87530 |
Infectious agent detection by nucleic acid (DNA or RNA); Herpes simplex virus, quantification |
|
|
87590 |
Infectious agent detection by nucleic acid (DNA or RNA); Neisseria gonorrhoeae, direct probe technique |
|
|
87591 |
Infectious agent detection by nucleic acid (DNA or RNA); Neisseria gonorrhoeae, amplified probe technique |
|
|
87592 |
Infectious agent detection by nucleic acid (DNA or RNA); Neisseria gonorrhoeae, quantification |
|
|
87623 |
Infectious agent detection by nucleic acid (DNA or RNA); Human Papillomavirus (HPV), low-risk types (e.g., 6, 11, 42, 43, 44) |
|
|
87624 |
Infectious agent detection by nucleic acid (DNA or RNA); Human Papillomavirus (HPV), high-risk types (e.g., 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) |
|
|
87625 |
Infectious agent detection by nucleic acid (DNA or RNA); Human Papillomavirus (HPV), types 16 and 18 only, includes type 45, if performed |
| 87660 | Infectious agent detection by nucleic acid (DNA or RNA); Trichomonas vaginalis, direct probe technique | |
| 87661 | Infectious agent detection by nucleic acid (DNA or RNA); Trichomonas vaginalis, amplified probe technique | |
|
|
87797 |
Infectious agent detection by nucleic acid (DNA or RNA), not otherwise specified; direct probe technique, each organism |
|
|
87798 |
Infectious agent detection by nucleic acid (DNA or RNA), not otherwise specified; amplified probe technique, each organism |
|
|
87799 |
Infectious agent detection by nucleic acid (DNA or RNA), not otherwise specified; quantification, each organism |
|
|
88341 |
Immunohistochemistry or immunocytochemistry, per specimen; each additional single antibody stain procedure (List separately in addition to code for primary procedure) |
|
|
88342 |
Immunohistochemistry or immunocytochemistry, per specimen; initial single antibody stain procedure |
|
|
88344 |
Immunohistochemistry or immunocytochemistry, per specimen; each multiplex antibody stain procedure |
|
|
0064U |
Antibody, Treponema pallidum, total and rapid plasma reagin (RPR), immunoassay, qualitative |
|
|
0065U |
Syphilis test, non-treponemal antibody, immunoassay, qualitative (RPR) |
|
|
0096U |
Human papillomavirus (HPV), high-risk types (i.e., 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68), male urine |
|
|
0167U |
Gonadotropin, chorionic (hCG), immunoassay with direct optical observation, blood |
|
|
0210U |
Syphilis test, non-treponemal antibody, immunoassay, quantitative (RPR) |
|
|
0500T |
Infectious agent detection by nucleic acid (DNA or RNA), Human Papillomavirus (HPV) for five or more separately reported high-risk HPV types (e.g., 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) (i.e., genotyping) |
|
|
G0432 |
Infectious agent antibody detection by enzyme immunoassay (EIA) technique, HIV-1 and/or HIV-2, screening |
|
|
G0433 |
Infectious agent antibody detection by enzyme-linked immunosorbent assay (ELISA) technique, HIV-1 and/or HIV-2, screening |
|
|
G0435 |
Infectious agent antibody detection by rapid antibody test, HIV-1 and/or HIV-2, screening |
|
|
G0472 |
Hepatitis C antibody screening, for individual at high risk and other covered indication(s) |
|
|
G0475 |
Hiv antigen/antibody, combination assay, screening |
|
|
G0499 |
Hepatitis b screening in non-pregnant, high risk individual includes hepatitis b surface antigen (HBSAG) followed by a neutralizing confirmatory test for initially reactive results, and antibodies to HBSAG (anti-HBs) and Hepatitis B core antigen (anti-HBc) |
|
|
S3645 |
HIV-1 antibody testing of oral mucosal transudate |
|
ICD-10 Codes |
Z51.81 |
Encounter for therapeutic drug level monitoring |
|
|
Z59.0 |
Homelessness |
|
|
Z59.9 |
Problem related to housing and economic circumstances, unspecified |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, Blue Cross Blue Shield Association technology assessment program (TEC) and other non-affiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2018 Forward
| 04/21/2023 | Interim review, policy updated to include T. vaginalis testing and STD testing for sexually active members under 18 years of age. Also updating for clarity and consistency. Updating description, notes, table of terminology, rationale, references and adding coding related to T. vaginalis.) |
| 11/07/2022 | Annual review, no change to policy intent. Reformatting policy for clarity. Adding table of terminology. Updating rationale, references, and coding. ) |
|
01/19/2022 |
Correcting typographical error in related policy number. |
|
10/01/2021 |
Annual review, updating policy to add and clarify coverage criteria related to herpes simplex testing and testing related to PrEP therapy. Also updating description, rationale and references. A also updating note 1 for clarity and updating coding |
|
10/24/2020 |
Annual review, no change to policy intent, minor revision in policy for clarity regarding age. also updating coding, rationale and references |
|
10/23/2020 |
Updating Coding. No other changes made to policy. |
|
10/29/2019 |
Annual review, adding policy statement regarding NAAT or PCR based testing for T. vaginalis and rapid identification of Trichomonas. Also updating coding. |
|
10/02/2019 |
Updating Annual review date. No other changes made |
|
07/16/2019 |
Interim review to expand ICD coding. No change to policy intent. |
|
01/08/2019 |
Interim review, adding not medically necessary criteria regarding immunoassay testing for herpes simplex virus-1. |
|
11/20/2018 |
New Policy |