Genetic Testing for Germline Mutations of the RET Proto-Oncogene - CAM 256
Description:
The rearranged during transfection (RET) proto-oncogene encodes a transmembrane receptor tyrosine kinase (Takahashi et al., 1985) that regulates a complex network of signal transduction pathways during development, survival, proliferation, differentiation, and migration of the enteric nervous system progenitor cells (Hedayati et al., 2016). Disruption of RET signaling by mutation, gene rearrangement, overexpression or transcriptional up-regulation of the RET gene is implicated in several human cancers (Plaza-Menacho et al., 2014), most commonly thyroid cancer, but also chronic myelomonocytic leukemia, acute myeloid leukemia, and lung, breast, pancreatic, and colon cancers (Gordon et al., 2018). Mutation of the RET gene in a germline cell results in an autosomal dominant hereditary cancer syndrome, multiple endocrine neoplasia type 2 (MEN2) characterized by medullary thyroid carcinoma (MTC), pheochromocytoma (PHEO), and primary parathyroid hyperplasia (PPTH) (Figlioli et al., 2013).
This policy covers genetic testing for germline variants in the RET gene. For information on testing of tumors for RET variants for guiding chemotherapy, see M2030 Testing for Targeted Therapy of Non-Small-Cell Lung Cancer and M2108 Molecular Markers in Fine Needle Aspirates of the Thyroid.
Regulatory Status
A search of the FDA database on 10/03/2020 using the term “genotyping” yielded 24 results. Additional tests may be considered laboratory developed tests (LDTs) if they are developed, validated and performed by individual laboratories. LDTs are regulated by the Centers for Medicare & Medicaid Services (CMS) as high-complexity tests under the Clinical Laboratory Improvement Amendments of 1988 (CLIA’88). As an LDT, the U.S. Food and Drug Administration has not approved or cleared this test; however, FDA clearance or approval is not currently required for clinical use.
Policy:
- Genetic testing for RET proto-oncogene point mutations is considered MEDICALLY NECESSARY when any of the following conditions are met:
- For individuals who are a member of a family with defined RET gene mutations.
- For individuals who are a member of a family that is known to be affected by inherited medullary thyroid cancer but the family has not previously been evaluated for RET mutations.
- For individuals with apparently sporadic medullary thyroid carcinoma (MTC).
- For individuals who are a first-degree relative (see Note 1) of individuals with sporadic MTC.
- For individuals with a diagnosis of MTC, a clinical diagnosis of multiple endocrine neoplasia type 2 (MEN2), or primary C-cell hyperplasia.
The following does not meet coverage criteria due to a lack of available published scientific literature confirming that the test(s) is/are required and beneficial for the diagnosis and treatment of a patient’s illness.
- For all other situations not discussed above, genetic testing for germline point mutations in the RET gene is considered NOT MEDICALLY NECESSARY.
NOTES:
Note 1: First-degree relatives include parents, full siblings, and children of the individual.
Table of Terminology
|
Term |
Definition |
|
A750T |
Alanine-750-threonine |
|
A883F |
Alanine-883-phenylalanine |
|
AKT |
AKT serine/threonine kinase 1 |
|
ALK |
ALK receptor tyrosine kinase |
|
AMP |
Association for Molecular Pathology |
|
ATA |
American Thyroid Association |
|
BTA |
The British Thyroid Association |
|
C634R |
Cysteine-634-arginine |
|
C634Y |
Cystine-634-tyrosine |
|
CAP |
The College of American Pathologists |
|
CDKN2A |
Cyclin dependent kinase inhibitor 2A |
|
CLA |
Cutaneous lichen amyloidosis |
|
CLIA’88 |
Clinical Laboratory Improvement Amendments of 1988 |
|
CMS |
Centers for Medicare & Medicaid Services |
|
DNA |
Deoxyribonucleic acid |
|
EGFR |
Epidermal growth factor receptor |
|
ESMO |
European Society for Medical Oncology |
|
ETA |
European Thyroid Association |
|
FDA |
Food and Drug Administration |
|
FFPE |
Formalin-fixed, paraffin-embedded |
|
FISH |
Fluorescent in situ hybridization |
|
FLT3 |
Fms related receptor tyrosine kinase 3 |
|
FMTC |
Familial medullary thyroid carcinoma |
|
HD |
MEN2A with Hirschsprung disease |
|
HPTH |
Primary hyperparathyroidism |
|
HSCR |
Hirschsprung's disease |
|
IASLC |
International Association for the Study of Lung Cancer |
|
IHC |
Immunohistochemistry |
|
L790F |
Leucine-790-phenylalanine |
|
LDTs |
Laboratory developed tests |
|
M918T |
Methionine-918-threonine |
|
MAPK |
Mitogen-activated protein kinase |
|
MEN |
Multiple endocrine neoplasia |
|
MEN2 |
Multiple endocrine neoplasia type 2 |
|
MEN2A |
Multiple endocrine neoplasia type 2A |
|
MEN2B |
Multiple endocrine neoplasia type 2B |
|
MTC |
Medullary thyroid carcinoma |
|
NAT2 |
N-acetyltransferase 2 |
|
NCCN |
National Comprehensive Cancer Network |
|
NGS |
Next-generation sequencing |
|
NHS |
National Health Services |
|
NSCLC |
Non-small cell lung cancer |
|
PTH |
Parathyroid hyperplasia |
|
PHEO |
Pheochromocytoma |
|
PPTH |
Primary parathyroid hyperplasia |
|
QOE |
Quality of evidence |
|
Q-PCR |
Quantitative polymerase chain reaction |
|
R387Q |
Arginine-387-glutamine |
|
R693H |
Arginine-693-histidine |
|
RET |
Rearranged during transfection |
|
ROS1 |
ROS proto-oncogene 1, receptor tyrosine kinase |
|
RT-PCR |
Real-time polymerase chain reaction |
|
SNP |
Single nucleotide polymorphism |
|
SOR |
Strength of recommendation |
|
V292M |
Valine-292-methionine |
|
V804M |
Valine-804-methionine |
|
VUS |
Variants of unknown significance |
Rationale
The RET gene encodes a receptor tyrosine kinase that transduces growth and differentiation signals from the glial cell-derived neurotrophic factor family of ligands (Saarma, 2001). RET is expressed in the neuroendocrine parafollicular C-cells of the thyroid gland, adrenal medulla, neurons, and other tissues (Takaya et al., 1996). Unlike loss of function mutations that inactivate tumor suppressor proteins, oncogenic RET mutations result in a gain of function, inducing ligand-independent autophosphorylation of the RET receptor (Santoro et al., 1995), uncontrolled activation of MAPK and phosphoinositide 3-kinase pathways, and ultimately uncontrolled growth and cell dedifferentiation (Hansford & Mulligan, 2000; Raue & Frank-Raue, 2018).
Oncogenic activation of the RET gene can result from either somatic or germline alterations. Activating germline point mutations in RET with autosomal dominant heritability have been identified as the primary initiating events causative of malignancy in C-cells of the thyroid gland (MTC) and other clinical presentations of MEN2 (Hansford & Mulligan, 2000; Mulligan, 2014). These mutations are identified in 98 – 100% of MEN2 cases (Raue & Frank-Raue, 2018; Romei et al., 2016), which are responsible for 25% of MTC cases overall (Raue & Frank-Raue, 2015). An estimated 64,000 patients are diagnosed with thyroid cancer in the United States annually, and 1 – 2% of these cases are due to MTC. The most common alterations in the RET proto-oncogene are missense gain-of-function mutations mainly located in the extracellular domain of the RET gene (exons 10 or 11) and in the RET tyrosine kinase domain (exons 13, 14, 15 and 16) (ATA, 2016).
Germline RET mutations are associated with clear genotype-phenotype correlations (Plaza-Menacho et al., 2014). These clinical phenotypes can be divided into two subclasses of MEN2: multiple endocrine neoplasia type 2A (MEN2A) including familial medullary thyroid carcinoma (FMTC) and MEN type 2B (MEN2B) (Jasim et al., 2011). Over 100 RET point mutations, duplications, insertions, deletions, and fusions have been identified in patients with MEN2A, with the C634R mutation in exon 11 being the most common mutation, whereas only two RET mutations have been identified in patients with MEN2B (mainly M918T, and rarely A883F) (Giani et al., 2020; Romei et al., 2018). New variants continue to be reported (Paragliola et al., 2018; Qi et al., 2018). For example, in a case study of a 7-year-old girl in Italy, a “de novo” new germline RET deletion in exon 11 was found to cause features of both MEN2B without PHEO (pheochromocytoma), but “with a pelvic plexiform neurofibroma and with HPTH (primary hyperparathyroidism), which is typical of MEN2A” (Giani et al., 2020).
MEN2A is characterized by MTC and variable rates of PHEO, PPTH or both, with RET mutations in codons 609, 611, 618, or 620 of exon 10 and codon 634 of exon 11. Subtypes of classical MEN2A include development of cutaneous lichen amyloidosis and Hirschsprung disease. Absence of any clinical finding other than MTC in at least four family members is classified as FMTC (Wells et al., 2015).
MEN2B is characterized by highly aggressive MTC, usually PHEO, but not PPTH, and may exhibit musculoskeletal abnormalities and developmental defects with RET mutations in codons 918 and 883 of exon 15 (Wells et al., 2015).
Figure 1: RET point mutations in MEN2A, MEN2B, and FMTC (Wells et al., 2013).
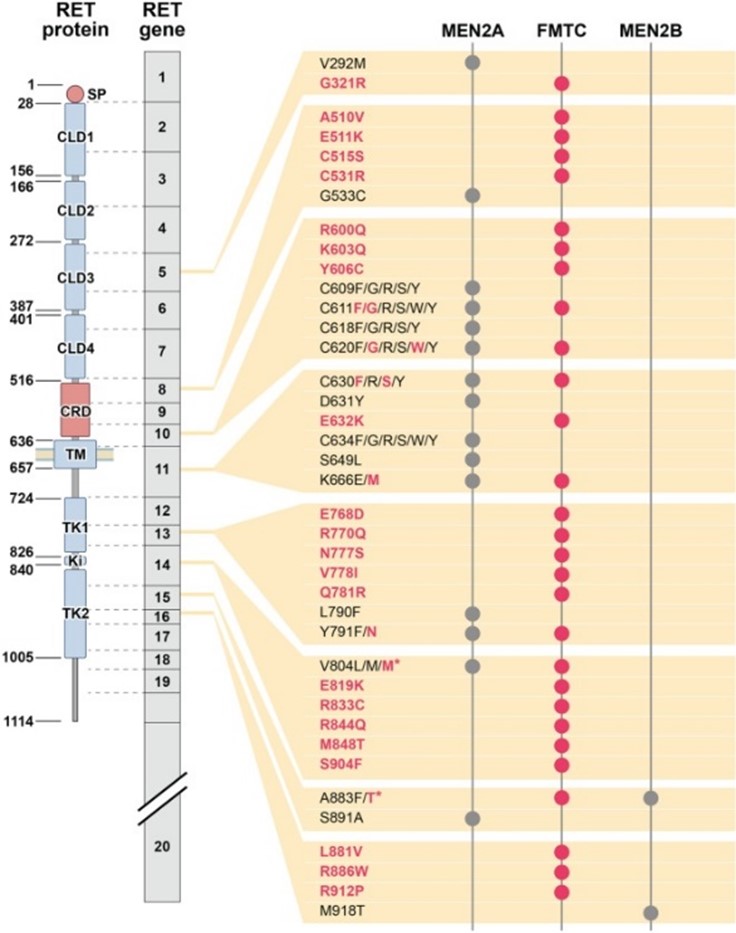
Table 1: Clinical expression of familial MTC-associated syndromes (Links et al., 2015).
|
|
FMTC (%) |
MEN2A (%) |
MEN2B (%) |
|
MTC |
100 |
100 |
100 |
|
Pheochromocytoma |
0 |
10 – 60 |
50 |
|
Hyperparathyroidism |
0 |
10 – 30 |
0 |
|
Marfanoid habitus |
0 |
0 |
100 |
|
Intestinal ganglioneuromatosis |
0 |
0 |
60 – 90 |
|
Mucosal neuromas |
0 |
0 |
70 – 100 |
Clinical Validity
The development of tyrosine kinase inhibitors that specifically target RET (Suyama & Iwase, 2018) has allowed for genetic analysis of RET germline mutations to become the standard of care in the initial workup for detecting germline mutations and familial risk and identifying targeted therapy in MTC (Ernani et al., 2016; Wells et al., 2015). Further, somatic RET rearrangements have recently been implicated in a variety of cancers, including chronic myelomonocytic leukemia; acute myeloid leukemia; and lung, breast, pancreatic, and colon cancers; a patient previously diagnosed with lung cancer underwent genomic profiling, and the identification of a RET point mutation associated with MTC allowed researchers to determine that this lung cancer diagnosis was incorrect (Gordon et al., 2018). A change in treatments proved to be very helpful for this patient. Other researchers have reported RET translocations in lung cancer cases, but they state that this is extremely rare (Zhao et al., 2016).
Guan et al. (2020) identified RET mutations in human epithelial ovarian cancer, providing another area of benefit from genetic testing of the RET gene for developing targeted therapies. Results showed that R693H and A750T mutants, in the juxtamembrane region and intracellular kinase domain, respectively, could promote the MAPK and AKT signaling pathway in ovarian cancer, and that the RET inhibitor vandetanib could decrease signal transduction and inhibit cancer growth (Guan et al., 2020).
Though activating variants in the receptor tyrosine kinase rearranged during transfection are known to be associated with MEN 2, not all variants are created equal. Hansen et al. (2021) explored the pathogenic role of the variant c.166C>A, p.Leu56Met in RET, which was identified in patients presenting short segment HSCR and more recently in two patients with MTC. Seven unrelated RET Leu56Met carriers from a Danish cohort were evaluated in the study, none of whom displayed evidence of MEN 2 or MTC based on the authors’ predefined criteria for MEN2B (“(i) the patient demonstrates more than one MEN 2 manifestation, including histologically verified MTC, histologically verified pheochromocytoma, histologically verified gastrointestinal or mucosal neuromas, histologically verified HSCR, biochemically verified PHPT, and clinically or histologically verified cutaneous lichen amyloidosis, or (ii) the patient has one MEN 2 manifestation and a relative with MTC and the RET Leu56Met variant”). Moreover, while known causative variants of MEN 2 such as p.Cys634Tyr and p.Met918Thr boast allele frequencies below 0.001%, the allele frequency for the Leu56Met variant computed from the in-house diagnostic cohort was much higher at 0.59%. These considerations, combined with the lack of family history for MEN 2, suggest that Leu56Met is “a common variant without clinical significance in the Danish population” and “is most likely a benign variant” (Hansen et al., 2021).
Researchers have also found in two RET L790F index patients that somatic RET variants were not responsible for the early onset and aggressiveness of MTC in a RET germline mutation carrier. Normally, variations in MTC presentation could be attributed to RET germline variants (Mathiesen et al., 2020). However, Mathiesen et al. (2020) found an FLT3 R387Q variant — FLT3 being a protein commonly found in hematopoietic malignancies — that could have been a genetic modifier instead.
Clinical Utility
The strong genotype-phenotype correlation of RET mutations makes genetic screening of significant value in diagnosis, prognosis, and management of MEN2 (Eng et al., 1996; Frank-Raue et al., 2010; Romei et al., 2015) and resultant MTC (Machens, Lorenz, et al., 2018), PHEO (Kimura et al., 2018), and PPTH. Each specific RET mutation correlates with MEN2 presentation, age at onset of MTC, and MTC aggressiveness (Brandi et al., 2001). Screening and early treatment of the manifestations of MEN2 can prevent metastasis of MTC and the morbidity and mortality caused by PHEO (Gagel et al., 1988; Makri et al., 2018). Moreover, screening has been associated with improved survivorship and outcomes (Raue et al., 2018). Based upon these genotype-phenotype correlations, RET mutations have been stratified into three risk levels based on the penetrance and aggressiveness of the MTC (Brandi et al., 2001; Wells et al., 2015). Consequently, mutation type should guide major management decisions, such as whether and when to perform thyroidectomy (Machens, Elwerr, et al., 2018; Machens, Lorenz, et al., 2018). Children in the highest risk category should undergo thyroidectomy in their first year of life, and perhaps even in their first months of life (Machens, Elwerr, et al., 2018; Machens, Lorenz, et al., 2018). Those with mutations in the high-risk category (codon 634 mutations) “should undergo thyroidectomy before reaching the age of 5 years” (Larouche et al., 2019). Annual biochemical screening in patients with a family history of FMTC or MEN2 can also be stopped in those patients who test negative for mutations (Wells et al., 2015).
Martins-Costa et al. (2018) performed RET genetic sequencing on exons 8, 10, 11, and 13 – 16 in 247 patients with MTC or who are at risk of developing MTC due to family history. Before genetic testing, 54 of these patients were diagnosed with sporadic disease and six were diagnosed with hereditary disease; after genetic testing, 31 patients were diagnosed with sporadic disease and 29 with hereditary disease (Martins-Costa et al., 2018). RET screening allowed several patients to be classified as hereditary who were initially diagnosed with sporadic MTC; 73 at-risk relatives were identified as mutation carriers, which will assist in long-term life and reproductive decisions (Martins-Costa et al., 2018).
A meta-analysis consisting of 438 Indian patients with MTC and 489 healthy controls of similar ages and genders was completed; all participants received molecular genetic testing including RET gene sequencing and SNP genotyping (Mishra et al., 2019). This study identified RET SNPs as a significant risk factor for developing hereditary MTC; CDKN2A and NAT2 SNPs with a significant risk of developing sporadic MTC (Mishra et al., 2019).
RET genetic screening was also provided to a total of 2031 Italian subjects; this included 1264 patients with sporadic MTC symptoms, 117 patients with hereditary MTC symptoms, and 650 relatives (Elisei et al., 2019). The researchers state, “A RET germline mutation was found in 115/117 (98.3%) hereditary and in 78/1264 (6.2%) apparently sporadic cases: in total, 42 distinct germline variants were found (Elisei et al., 2019).” This thereby underscores the significance of genetic screening in unsuspected MEN2 families. Sporadic MTC cases were present most commonly with a V804M mutation, and all M918T mutations were de novo “and exclusively associated with MEN2B” (Elisei et al., 2019). These researchers also identified several variants of unknown significance (VUS).
Similarly, a paper by Milićević et al. (2021) focused on examining the crude annual incidence rate of MTC and RET mutation frequency. The study involved Slovenian patients at the Institute of Oncology Ljubljana from 1995 to 2015 and involved their family members who participated in genetic counseling and testing there. It was found that among 143 patients with MTC, 37 (25.9%) harbored a germline RET mutation, and said mutations were uncovered in exons 10, 11, 13, 14, and 16. Also, the researchers noted that “RET germline mutations are quite commonly discovered even in the apparently sporadic form of the disease”, such that 14.2% of patients with a negative family history were presented with them. It was also reported that the most frequent RET germline mutations were found on codons 634 and 618 (30.0%) and exon 11 was the most frequently altered, though mutations of codons 790, 804, and 918 were observed in smaller but noticeable percentages (25.0%, 10.0%, and 5.0%, respectively). However, despite the low compliance of family members resulting in a smaller pool of participants, the authors extol the use of genetic counseling in this case, as “Annual incidence increase and nation-specific frequency of discovered RET mutations justify the continuation of gene counseling and testing of MTC patients in Slovenia” (Milićević et al., 2021).
Another study by Fussey et al. (2021) aimed to lend their perspective on the diagnostic potential of RET genetic testing. Between 1997 and 2018, the Exeter Genomics Laboratory at the Royal Devon and Exeter NHS Foundation Trust collected information on 1058 index patients with MTC and other MEN2-related clinical features. They found that in total, 92 of the 766 UK patients with MTC were harboring a germline RET pathogenic variant, and that variants in 10, 11, and 14 comprised its bulk, with codons 634 in exon 11 and 804 in exon 14 being most often affected. As such, the researchers believe that “the use of somatic RET analysis to confirm the diagnosis of sporadic MTC in patients with no identified germline RET variants may be a useful adjunct both in terms of reassuring family members about the lack of a heritable pathogenic germline variant, and risk-stratifying sporadic tumours based on somatic variants” (Fussey et al., 2021).
RET genetic screening could also disclose new variants with their respective phenotypes. Yang et al. (2020) described a compound C634Y/V292M transmutation in a northern Chinese family that was associated with a more aggressive clinical presentation. Carriers of this variant had bilateral MTC with PHEO or lymph node metastasis with faster cell growth (cell growth speed identified in vitro). On the other hand, carriers of the V292M variant were asymptomatic, and carriers of the C634Y mutation only had elevated calcitonin (Yang et al., 2020). This has demonstrated the striking variability in MTC clinical presentation based on RET gene variants, making it critical to aid in any future potential treatment regimen.
Though activating variants in the receptor tyrosine kinase rearranged during transfection are known to be associated with MEN 2, not all variants are created equal. Hansen et al. (2021) explored the pathogenic role of the variant c.166C>A, p.Leu56Met in RET, which was identified in patients presenting short segment HSCR and more recently in two patients with MTC. Seven unrelated RET Leu56Met carriers from a Danish cohort were evaluated in the study, none of whom displayed evidence of MEN 2 or MTC based on the authors’ predefined criteria for MEN2B (“(i) the patient demonstrates more than one MEN 2 manifestation, including histologically verified MTC, histologically verified pheochromocytoma, histologically verified gastrointestinal or mucosal neuromas, histologically verified HSCR, biochemically verified PHPT, and clinically or histologically verified cutaneous lichen amyloidosis, or (ii) the patient has one MEN 2 manifestation and a relative with MTC and the RET Leu56Met variant”). Moreover, while known causative variants of MEN 2 such as p.Cys634Tyr and p.Met918Thr boast allele frequencies below 0.001%, the allele frequency for the Leu56Met variant computed from the in-house diagnostic cohort was much higher at 0.59%. These considerations, combined with the lack of family history for MEN 2, suggest that Leu56Met is “a common variant without clinical significance in the Danish population” and “is most likely a benign variant” (Hansen et al., 2021).
European Society for Medical Oncology (ESMO)
The ESMO has published clinical practice guidelines on diagnosis, treatment, and follow-up of thyroid cancer, stating that “All patients with MTC should be offered genetic counselling and screened for germline RET mutations” (Filetti et al., 2020). Filetti et al. (2020) also stated that “screening for somatic RET mutations is only recommended if RET inhibitor therapy is planned.”
In 2021, ESMO Translational Research and Precision Medicine Working Group expanded upon the algorithm to identify patients eligible for anti-RET therapy across three scenarios based on the malignancies. The recommendations are captured below:
“Scenario A: Patients affected by NSCLC, non-MTC or other solid tumours, with available formalin-fixed, paraffin-embedded (FFPE) specimen need to be screened for detection of RET fusion. If NGS is not available, FISH or RT-PCR is indicated in NSCLC and non-MTC, depending on local availability, cost and/or amount of tumour cells. In case of a negative test result, it is recommended to perform an NGS panel. It should be noted, however, that the recent ESMO recommendations suggest using multigene NGS to assess NSCLC level I alterations, including RET fusions.
In addition to RET fusion testing, patients affected by other solid tumours may need to be tested also for RET mutation according to the results of pending clinical trials. This can be done preferably by NGS; if this is not available, Q-PCR can be used. In none of the above cases is RET IHC testing recommended.
Scenario B: For patients affected by NSCLC, non-MTC or other solid tumours whose FFPE specimens are not available or are exhausted, we suggest performing a liquid biopsy (cell-free nucleic acid NGS panel) to test for RET alteration.
It is important to notice that if an RET alteration is not detected by liquid biopsy, then tumour tissue testing is still required to definitively exclude the possibility of an RET fusion.
Scenario C: Patients affected by MTC need to be screened for detection of RET mutation. They should be referred to genetic counselling in order to study the presence of MEN syndrome or FMTC. Mutations in the RET gene, in fact, are canonical in hereditary MTC, but can be found also in sporadic MTC. A Q-PCR or NGS can be carried out on sputum or blood of the patient. In case of known familial RET mutation, a simple Sanger test can be carried out on blood leukocyte DNA. If a germline RET mutation is present, family counselling is indicated. In case of absence of germline RET mutations, if the patient with MTC becomes metastatic, a Q-PCR of NGS analysis on FFPE tissue specimens from metastatic site of disease should be carried out in order to confirm or reject the presence of this alteration.
In none of the above cases is RET IHC testing recommended” (Belli et al., 2021).
American Thyroid Association (ATA)
The ATA published revised guidelines (Wells et al., 2015)i n which state that:
- “Initial testing for patients with MEN2A phenotype is either a single or multi-tiered analysis to detect RET mutations in exon 10 (codons 609, 611, 618, and 620), exon 11 (codons 630 and 634), and exons 8, 13, 14, 15 and 16." Grade B Recommendation.
- Initial testing for patients with MEN2B phenotype should be tested for the RET codon M918T mutation (exon 16), and if negative, the RET codon A883F mutation (exon 15).
- “Sequencing of the entire coding region should be reserved for situations in which no RET mutation is identified or there is a discrepancy between the MEN2 phenotype and the expected genotype." Grade B Recommendation
- Patients with the MEN2B phenotype should be tested for the RET codon M918T mutation (exon 16), and if negative, the RET codon A883F mutation (exon 15). If there are no mutations identified in these two exons the entire RET coding region should be sequenced. Grade B Recommendation
- Patients with presumed sporadic MTC should have genetic testing to detect a germline RET mutation. If a RET mutation is found the patient should have genetic testing. Grade B Recommendation
- In very rare families who meet the clinical criteria for MEN2A or 2B, despite negative sequencing of the entire RET coding region, the relatives at risk should be periodically screened by conventional methods for MTC, PHEO, and HPTH. After the initial evaluation, screening should continue at 1- to 3-year intervals. Grade C Recommendation
- Genetic counseling and genetic testing for RET germline mutations should be offered to
- First-degree relatives of patients with proven hereditary MTC,
- Parents whose infants or young children have the classic phenotype of MEN2B,
- Patients with CLA [cutaneous lichen amyloidosis], and
- Infants or young children with HD and exon 10 RET germline mutations, and adults with MEN2A and exon 10 mutations who have symptoms suggestive of HD” (Wells et al., 2015)
National Comprehensive Cancer Network
NCCN guidelines for neuroendocrine and adrenal tumors (NCCN, 2022a) recommends that for diagnosis of or clinical suspicion of MEN2, genetic counseling and RET genetic testing should be offered in the following cases:
- “Individuals with MTC or primary C-cell hyperplasia or a clinical diagnosis of MEN2."
- “At-risk relative of an individual with a known germline RET mutation at a very young age.”
- “50% of cases have de novo RET mutations; therefore, even if a family history is not suggestive of a hereditary syndrome, genetic testing for RET mutations should still be performed on the affected individual.”
- “All patients with MTC should be tested for germline mutation of the RET oncogene even if the family history is not suggestive of a hereditary syndrome, because about 50% of patients with presumed sporadic MTC have a de novo germline mutation” (NCCN, 2022a).
NCCN Guidelines for Thyroid Carcinoma (NCCN, 2022b) stated that as part of the additional workup after medullary thyroid carcinoma is identified by initial thyroid surgery, a “Screen for germline RET proto-oncogene mutations (exons 10, 11, 13 – 16)” and “Germline mutation should prompt specific mutation testing in subsequent family members and genetic counseling” (NCCN, 2022b).
The College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and the Association for Molecular Pathology (AMP)
The 2013 guidelines from CAP, IASLC and AMP for molecular testing in lung cancer patients have been updated in 2018; new recommendations state that RET testing is approved in lung cancer specimens “as part of larger testing panels performed either initially or when routine EGFR, ALK, and ROS1 testing are negative” because “RET molecular testing is not recommended as a routine stand-alone assay outside the context of a clinical trial” (Lindeman et al., 2018).
The British Thyroid Association (BTA)
Regarding MTC, the BTA has stated that “In all confirmed cased of MTC, RET mutation analysis to establish the possible genetic basis for the disease within an individual or kindred, should be performed even in the absence of a positive family history.” Other key recommendations to consider before genetic testing are listed below.
- “Because of the possibility of heritable disease, every case of MTC should be offered genetic testing."
- “Testing should always begin with the affected individual, if they are available."
- “If the affected individual is not available, the decision and strategy for testing should be discussed with the clinical genetics service."
- “Before blood is taken, a clear explanation must be given of the nature of the test, the possible outcomes, and of the implications of a positive or negative result for the individual and the family. This explanation should be recorded in the case notes for each individual” (Perros et al., 2014).
European Thyroid Association (ETA)
The ETA Executive Committee in 2012 issued guidelines specifically devoted to RET genetic screening for patients affected by medullary thyroid cancers (MTC) with reference to three different phenotypes: multiple endocrine neoplasia types 2A and 2B and familial MTC (FMTC). In their attempt to improve the quality of care for patients and families with MTC, they made recommendations with varying valences, grading their own recommendations by the quality of evidence (QOE), which is indicated by plus signs at three levels, and the strength of recommendation (SOR) score, indicated by a 1 (strong recommendation for or against) or a 2 (“ weak recommendation or a suggestion that may not be appropriate for every patient, depending on the context, patient values, and preferences”). The relevant parts of the guidelines are
From Recommendation 4:
“(a) Exons 5, 8, 10, 11, 13, 14, 15, and 16 should always be analyzed starting from the most likely involved in the presenting syndrome (i.e. exon 10 in MEN 2A, exon 16 in MEN 2B, etc.). DNA from MTC patients with a strong suggestion of familial disease should also be analyzed for all other uncommonly mutated exons when those listed above are negative… (QOE = +++; SOR = score 1).”
(b) Subjects belonging to the few families (2 – 5% of all MEN 2/FMTC families) with clinically evident MEN 2/FMTC features but lacking evidence of an associated germline RET mutation should be followed up annually by measurement of basal serum Ct, metanephrines, calcium, and PTH. Neck and abdomen ultrasound could also be useful although not absolutely required if biochemical tests are still negative (QOE = ++; SOR = score 1).
(c) Once a germline RET mutation has been identified, all first-degree relatives and other relevant family members should be screened for the specific causative mutation (QOE = +++; SOR = score 1).
(d) Once a germline RET mutation has been discovered, further sequencing adds little, although some cases of double or triple RET mutations have been reported. These peculiar cases would likely be missed if RET screening was stopped when the first mutation was identified. The completeness of RET gene analysis is particularly indicated when the first identified mutation is rare and with a low or null transforming ability. These rare cases should be referred to specialized tertiary centers for a complete characterization of the mutation and its relationship with the disease (QOE = ++; SOR = score 2).
(e) Family members negative for the mutation are not at risk for the development of MTC and their children are not at risk either. Such individuals should be reassured and do not require further investigation or follow-up (QOE = +++; SOR = score 1).”
From Recommendation 5:
“(a) All patients with either apparently sporadic or familial MTC should be screened for germline RET mutations (QOE = +++; SOR = score 1).”
From Recommendation 6:
“(a) Patients with bilateral PHEO [pheochromocytoma] should be considered for RET genetic screening when other MEN 2A endocrinopathies (MTC and/or PHPT [primary hyperparathyroidism]) or CLA are present in the same subject or if a family history of MEN 2A or 2B is present. The screening becomes mandatory if the basal serum Ct is above the normal range, independently of the presence of other MEN 2A endocrinopathies (QOE = ++; SOR = score 1).
(b) Patients with multiple adenomatosis of the parathyroid glands and PHPT should be considered for RET genetic screening if other MEN 2A endocrinopathies (MTC and/or PHEO) or CLA are present in the same subject or if a family history of MEN 2A is present. Also in this case, RET genetic screening must be performed if basal serum Ct levels are above the normal range, even if no other MEN 2A endocrinopathies are diagnosed (QOE = ++; SOR = score 1).
(c) Subjects with CLA should be investigated clinically and should undergo genetic testing for MEN 2A. In particular RET codon 634 in exon 11 should be analyzed (QOE = +++; SOR = score 1).
(d) Patients with Hirschsprung disease should have RET genetic screening for mutations involved in Hirschsprung disease, but analyses performed specifically to investigate an association with MEN 2 should be limited to exon 10 (QOE = +++; SOR = score 1).”
References:
- ATA. (2016). Cancer of the Thyroid. https://www.thyroid.org/wp-content/uploads/patients/brochures/medullary-thyroid-cancer-brochure.pdf
- Belli, C., Penault-Llorca, F., Ladanyi, M., Normanno, N., Scoazec, J. Y., Lacroix, L., Reis-Filho, J. S., Subbiah, V., Gainor, J. F., Endris, V., Repetto, M., Drilon, A., Scarpa, A., André, F., Douillard, J. Y., & Curigliano, G. (2021). ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann Oncol, 32(3), 337-350. https://doi.org/10.1016/j.annonc.2020.11.021
- Brandi, M. L., Gagel, R. F., Angeli, A., Bilezikian, J. P., Beck-Peccoz, P., Bordi, C., Conte-Devolx, B., Falchetti, A., Gheri, R. G., Libroia, A., Lips, C. J., Lombardi, G., Mannelli, M., Pacini, F., Ponder, B. A., Raue, F., Skogseid, B., Tamburrano, G., Thakker, R. V., . . . Marx, S. J. (2001). Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab, 86(12), 5658-5671. https://doi.org/10.1210/jcem.86.12.8070
- Elisei, R., Tacito, A., Ramone, T., Ciampi, R., Bottici, V., Cappagli, V., Viola, D., Matrone, A., Lorusso, L., Valerio, L., Giani, C., Campopiano, C., Prete, A., Agate, L., Molinaro, E., & Romei, C. (2019). Twenty-Five Years Experience on RET Genetic Screening on Hereditary MTC: An Update on The Prevalence of Germline RET Mutations. Genes (Basel), 10(9). https://doi.org/10.3390/genes10090698
- Eng, C., Clayton, D., Schuffenecker, I., Lenoir, G., Cote, G., Gagel, R. F., van Amstel, H. K., Lips, C. J., Nishisho, I., Takai, S. I., Marsh, D. J., Robinson, B. G., Frank-Raue, K., Raue, F., Xue, F., Noll, W. W., Romei, C., Pacini, F., Fink, M., . . . et al. (1996). The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA, 276(19), 1575-1579. https://www.ncbi.nlm.nih.gov/pubmed/8918855
- Ernani, V., Kumar, M., Chen, A. Y., & Owonikoko, T. K. (2016). Systemic treatment and management approaches for medullary thyroid cancer. Cancer Treat Rev, 50, 89-98. https://doi.org/10.1016/j.ctrv.2016.09.006
- Figlioli, G., Landi, S., Romei, C., Elisei, R., & Gemignani, F. (2013). Medullary thyroid carcinoma (MTC) and RET proto-oncogene: mutation spectrum in the familial cases and a meta-analysis of studies on the sporadic form. Mutat Res, 752(1), 36-44. https://doi.org/10.1016/j.mrrev.2012.09.002
- Filetti, Durante, Hartl, Leboulleux, Locati, Newbold, Papotti, & Berruti. (2020, April 16 2020). Thyroid Cancer - ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. European Society for Medical Oncology (ESMO). Retrieved October 5 from https://www.esmo.org/content/download/286312/5688706/1/Clinical-Practice-Guidelines-Slide-Set-Thyroid-Cancer.pdf
- Frank-Raue, K., Rondot, S., & Raue, F. (2010). Molecular genetics and phenomics of RET mutations: Impact on prognosis of MTC. Mol Cell Endocrinol, 322(1-2), 2-7. https://doi.org/10.1016/j.mce.2010.01.012
- Fussey, J. M., Smith, J. A., Cleaver, R., Bowles, C., Ellard, S., Vaidya, B., & Owens, M. (2021). Diagnostic RET genetic testing in 1,058 index patients: A UK centre perspective. Clin Endocrinol (Oxf), 95(2), 295-302. https://doi.org/10.1111/cen.14395
- Gagel, R. F., Tashjian, A. H., Jr., Cummings, T., Papathanasopoulos, N., Kaplan, M. M., DeLellis, R. A., Wolfe, H. J., & Reichlin, S. (1988). The clinical outcome of prospective screening for multiple endocrine neoplasia type 2a. An 18-year experience. N Engl J Med, 318(8), 478-484. https://doi.org/10.1056/NEJM198802253180804
- Giani, C., Ramone, T., Romei, C., Ciampi, R., Tacito, A., Valerio, L., Agate, L., Ugolini, C., Marinò, M., Basolo, F., Franchi, A., Borsari, S., Michelucci, A., Selli, C., Materazzi, G., Cetani, F., & Elisei, R. (2020). A New MEN2 Syndrome with Clinical Features of Both MEN2A and MEN2B Associated with a New RET Germline Deletion. Case Rep Endocrinol, 2020, 4147097. https://doi.org/10.1155/2020/4147097
- Gordon, E. J., Parker, D., Barth, K., Pena, J., Elvin, J. A., DeLeon, T., & Karlin, N. J. (2018). Genomic Profiling Reveals Medullary Thyroid Cancer Misdiagnosed as Lung Cancer. Case Rep Oncol, 11(2), 399-403. https://doi.org/10.1159/000490238
- Guan, L., Li, Z., Xie, F., Pang, Y., Zhang, C., Tang, H., Zhang, H., Chen, C., Zhan, Y., Zhao, T., Jiang, H., Jia, X., Wang, Y., & Lu, Y. (2020). Oncogenic and drug-sensitive RET mutations in human epithelial ovarian cancer. J Exp Clin Cancer Res, 39(1), 53. https://doi.org/10.1186/s13046-020-01557-3
- Hansen, A. R., Borgwardt, L., Rasmussen Å, K., Godballe, C., Poulsen, M. M., Vieira, F. G., Mathiesen, J. S., & Rossing, M. (2021). Germline RET Leu56Met Variant Is Likely Not Causative of Multiple Endocrine Neoplasia Type 2. Front Endocrinol (Lausanne), 12, 764512. https://doi.org/10.3389/fendo.2021.764512
- Hansford, J. R., & Mulligan, L. M. (2000). Multiple endocrine neoplasia type 2 and RET: from neoplasia to neurogenesis. J Med Genet, 37(11), 817-827. https://www.ncbi.nlm.nih.gov/pubmed/11073534
- Hedayati, M., Zarif Yeganeh, M., Sheikholeslami, S., & Afsari, F. (2016). Diversity of mutations in the RET proto-oncogene and its oncogenic mechanism in medullary thyroid cancer. Crit Rev Clin Lab Sci, 53(4), 217-227. https://doi.org/10.3109/10408363.2015.1129529
- Jasim, S., Ying, A. K., Waguespack, S. G., Rich, T. A., Grubbs, E. G., Jimenez, C., Hu, M. I., Cote, G., & Habra, M. A. (2011). Multiple endocrine neoplasia type 2B with a RET proto-oncogene A883F mutation displays a more indolent form of medullary thyroid carcinoma compared with a RET M918T mutation. Thyroid, 21(2), 189-192. https://doi.org/10.1089/thy.2010.0328
- Kimura, N., Takekoshi, K., & Naruse, M. (2018). Risk Stratification on Pheochromocytoma and Paraganglioma from Laboratory and Clinical Medicine. J Clin Med, 7(9). https://doi.org/10.3390/jcm7090242
- Larouche, V., Akirov, A., Thomas, C. M., Krzyzanowska, M. K., & Ezzat, S. (2019). A primer on the genetics of medullary thyroid cancer. Curr Oncol, 26(6), 389-394. https://doi.org/10.3747/co.26.5553
- Lindeman, N. I., Cagle, P. T., Aisner, D. L., Arcila, M. E., Beasley, M. B., Bernicker, E. H., Colasacco, C., Dacic, S., Hirsch, F. R., Kerr, K., Kwiatkowski, D. J., Ladanyi, M., Nowak, J. A., Sholl, L., Temple-Smolkin, R., Solomon, B., Souter, L. H., Thunnissen, E., Tsao, M. S., . . . Yatabe, Y. (2018). Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med, 142(3), 321-346. https://doi.org/10.5858/arpa.2017-0388-CP
- Links, T. P., Verbeek, H. H., Hofstra, R. M., & Plukker, J. T. (2015). Endocrine tumours: progressive metastatic medullary thyroid carcinoma: first- and second-line strategies. Eur J Endocrinol, 172(6), R241-251. https://doi.org/10.1530/EJE-14-0726
- Machens, A., Elwerr, M., Lorenz, K., Weber, F., & Dralle, H. (2018). Long-term outcome of prophylactic thyroidectomy in children carrying RET germline mutations. Br J Surg, 105(2), e150-e157. https://doi.org/10.1002/bjs.10746
- Machens, A., Lorenz, K., Weber, F., & Dralle, H. (2018). Genotype-specific progression of hereditary medullary thyroid cancer. Hum Mutat, 39(6), 860-869. https://doi.org/10.1002/humu.23430
- Makri, A., Akshintala, S., Derse-Anthony, C., Del Rivero, J., Widemann, B., Stratakis, C. A., Glod, J., & Lodish, M. (2018). Pheochromocytoma in children and adolescents with multiple endocrine neoplasia type 2B. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2018-00705
- Martins-Costa, M. C., Lindsey, S. C., Cunha, L. L., Carreiro-Filho, F. P., Cortez, A. P., Holanda, M. E., Farias, J. W. M., Lima, S. B., Ferreira, L. A. A., Maia Filho, P. C., Camacho, C. P., Furuzawa, G. K., Kunii, I. S., Dias-da-Silva, M. R., Martins, J. R. M., & Maciel, R. M. B. (2018). A pioneering RET genetic screening study in the State of Ceara, Brazil, evaluating patients with medullary thyroid cancer and at-risk relatives: experience with 247 individuals. Arch Endocrinol Metab, 62(6), 623-635. https://doi.org/10.20945/2359-3997000000088
- Mathiesen, J. S., Nielsen, S. G., Rasmussen Å, K., Kiss, K., Wadt, K., Hermann, A. P., Nielsen, M. F., Larsen, S. R., Brusgaard, K., Frederiksen, A. L., Godballe, C., & Rossing, M. (2020). Variability in Medullary Thyroid Carcinoma in RET L790F Carriers: A Case Comparison Study of Index Patients. Front Endocrinol (Lausanne), 11, 251. https://doi.org/10.3389/fendo.2020.00251
- Milićević, S., Bergant, D., Žagar, T., & Perić, B. (2021). Crude annual incidence rate of medullary thyroid cancer and RET mutation frequency. Croat Med J, 62(2), 110-119. https://doi.org/10.3325/cmj.2021.62.110
- Mishra, V., Kowtal, P., Rane, P., & Sarin, R. (2019). Genetic risk association of CDKN1A and RET gene SNPs with medullary thyroid carcinoma: Results from the largest MTC cohort and meta-analysis. Cancer Med, 8(13), 6151-6161. https://doi.org/10.1002/cam4.2443
- Mulligan, L. M. (2014). RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer, 14(3), 173-186. https://doi.org/10.1038/nrc3680
- NCCN. (2022a, May 23, 2022). Neuroendocrine and Adrenal Tumors Version 1.2022 - May 23, 2022. Retrieved October 26 from https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- NCCN. (2022b, May 5, 2022). Thyroid Carcinoma Version 2.2022 - May 5, 2022. Retrieved October 26, 2022 from https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf
- Paragliola, R. M., Lovicu, R. M., Papi, G., Capoluongo, E., Minucci, A., Canu, G., Pontecorvi, A., & Corsello, S. M. (2018). Medullary Thyroid Carcinoma With Exon 2 p.L56M RET Variant: Clinical Particular Features in Two Patients. Front Endocrinol (Lausanne), 9, 398. https://doi.org/10.3389/fendo.2018.00398
- Perros, P., Colley, S., Boelaert, K., Evans, C., Evans, R., Gerrard, G., Gilbert, J., Harrison, B., Johnson, S., Giles, T., Moss, L., Lewington, V., Newbold, K., Taylor, J., Thakker, R., Watkinson, J., & Williams, G. (2014). British Thyroid Association Guidelines for the Management of Thyroid Cancer. https://onlinelibrary.wiley.com/doi/pdf/10.1111/cen.12515
- Plaza-Menacho, I., Mologni, L., & McDonald, N. Q. (2014). Mechanisms of RET signaling in cancer: current and future implications for targeted therapy. Cell Signal, 26(8), 1743-1752. https://doi.org/10.1016/j.cellsig.2014.03.032
- Qi, X. P., Peng, J. Z., Yang, X. W., Zao, Z. L., Yu, X. H., Fang, X. D., Zhang, D. H., & Zhao, J. Q. (2018). The RET C611Y mutation causes MEN 2A and associated cutaneous. Endocr Connect, 7(9), 998-1005. https://doi.org/10.1530/EC-18-0220
- Raue, F., Dralle, H., Machens, A., Bruckner, T., & Frank-Raue, K. (2018). Long-Term Survivorship in Multiple Endocrine Neoplasia Type 2B Diagnosed Before and in the New Millennium. J Clin Endocrinol Metab, 103(1), 235-243. https://doi.org/10.1210/jc.2017-01884
- Raue, F., & Frank-Raue, K. (2015). Epidemiology and Clinical Presentation of Medullary Thyroid Carcinoma. Recent Results Cancer Res, 204, 61-90. https://doi.org/10.1007/978-3-319-22542-5_3
- Raue, F., & Frank-Raue, K. (2018). Update on Multiple Endocrine Neoplasia Type 2: Focus on Medullary Thyroid Carcinoma. J Endocr Soc, 2(8), 933-943. https://doi.org/10.1210/js.2018-00178
- Romei, C., Ciampi, R., Casella, F., Tacito, A., Torregrossa, L., Ugolini, C., Basolo, F., Materazzi, G., Vitti, P., & Elisei, R. (2018). RET mutation heterogeneity in primary advanced medullary thyroid cancers and their metastases. Oncotarget, 9(11), 9875-9884. https://doi.org/10.18632/oncotarget.23986
- Romei, C., Ciampi, R., & Elisei, R. (2016). A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol, 12(4), 192-202. https://doi.org/10.1038/nrendo.2016.11
- Romei, C., Tacito, A., Molinaro, E., Agate, L., Bottici, V., Viola, D., Matrone, A., Biagini, A., Casella, F., Ciampi, R., Materazzi, G., Miccoli, P., Torregrossa, L., Ugolini, C., Basolo, F., Vitti, P., & Elisei, R. (2015). Twenty years of lesson learning: how does the RET genetic screening test impact the clinical management of medullary thyroid cancer? Clin Endocrinol (Oxf), 82(6), 892-899. https://doi.org/10.1111/cen.12686
- Saarma, M. (2001). GDNF recruits the signaling crew into lipid rafts. Trends Neurosci, 24(8), 427-429. https://www.ncbi.nlm.nih.gov/pubmed/11476867
- Santoro, M., Carlomagno, F., Romano, A., Bottaro, D. P., Dathan, N. A., Grieco, M., Fusco, A., Vecchio, G., Matoskova, B., Kraus, M. H., & et al. (1995). Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science, 267(5196), 381-383. https://www.ncbi.nlm.nih.gov/pubmed/7824936
- Suyama, K., & Iwase, H. (2018). Lenvatinib: A Promising Molecular Targeted Agent for Multiple Cancers. Cancer Control, 25(1), 1073274818789361. https://doi.org/10.1177/1073274818789361
- Takahashi, M., Ritz, J., & Cooper, G. M. (1985). Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell, 42(2), 581-588. https://www.ncbi.nlm.nih.gov/pubmed/2992805
- Takaya, K., Yoshimasa, T., Arai, H., Tamura, N., Miyamoto, Y., Itoh, H., & Nakao, K. (1996). Expression of the RET proto-oncogene in normal human tissues, pheochromocytomas, and other tumors of neural crest origin. J Mol Med (Berl), 74(10), 617-621. https://www.ncbi.nlm.nih.gov/pubmed/8912182
- Wells, S. A., Jr., Asa, S. L., Dralle, H., Elisei, R., Evans, D. B., Gagel, R. F., Lee, N., Machens, A., Moley, J. F., Pacini, F., Raue, F., Frank-Raue, K., Robinson, B., Rosenthal, M. S., Santoro, M., Schlumberger, M., Shah, M., Waguespack, S. G., & American Thyroid Association Guidelines Task Force on Medullary Thyroid, C. (2015). Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid, 25(6), 567-610. https://doi.org/10.1089/thy.2014.0335
- Wells, S. A., Jr., Pacini, F., Robinson, B. G., & Santoro, M. (2013). Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab, 98(8), 3149-3164. https://doi.org/10.1210/jc.2013-1204
- Yang, Z., Qi, X., Gross, N., Kou, X., Bai, Y., Feng, Y., Wang, B., Zafereo, M. E., Li, G., Sun, C., Li, H., Chen, X., & Huang, Z. (2020). The synergy of germline C634Y and V292M RET mutations in a northern Chinese family with multiple endocrine neoplasia type 2A. J Cell Mol Med. https://doi.org/10.1111/jcmm.15922
- Zhao, W., Choi, Y. L., Song, J. Y., Zhu, Y., Xu, Q., Zhang, F., Jiang, L., Cheng, J., Zheng, G., & Mao, M. (2016). ALK, ROS1 and RET rearrangements in lung squamous cell carcinoma are very rare. Lung Cancer, 94, 22-27. https://doi.org/10.1016/j.lungcan.2016.01.011
Coding Section
| Codes | Number | Description |
| CPT | 81404 |
Molecular pathology procedure, Level 5 Gene: RET (ret proto-oncogene) (e.g., multiple endocrine neoplasia, type 2B and familial medullary thyroid carcinoma), common variants (eg, M918T, 2647_2648delinsTT, A883F) |
| 81405 |
Molecular pathology procedure, Level 6 Gene: RET (ret proto-oncogene) (e.g., multiple endocrine neoplasia, type 2A and familial medullary thyroid carcinoma), targeted sequence analysis (e.g., exons 10, 11, 13 – 16) |
|
| 81406 |
Molecular pathology procedure, Level 7 Gene: MUTYH (mutY homolog [E.coli]) (e.g., MYH-associated polyposis), full gene sequence |
|
| HCPCS | S3840 | DNA analysis for germline mutations of the ret proto-oncogene for susceptibility to multiple endocrine neoplasia type 2 |
| ICD-10-CM (effective 10/01/15) | C73 | Malignant neoplasm of thyroid gland |
| C7989 | Secondary malignant neoplasm of other specified sites | |
| D093 | Carcinoma in situ of Thyroid and other Endocrine Glands | |
| D098 | Carcinoma in situ of other specified sites | |
| Z85850 | Personal history of malignant neoplasm of Thyroid | |
| Z808 | Family History of Malignant neoplasm of other organs or systems | |
| ICD-10-PCS (effective 10/01/15) |
ICD-10 codes are only used for inpatient services. There is no specific ICD-10-PCS code for this procedure. |
|
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, Blue Cross Blue Shield Association technology assessment program (TEC) and other non-affiliated technology evaluation centers, reference to federal regulations, other plan medical policies and accredited national guidelines.
"Current Procedural Terminology© American Medical Association. All Rights Reserved"
History From 2013 Forward
| 01/27/2023 | Annual review, no change to policy intent. Policy verbiage updated for clarity including addition of note #1. Also, updating description, rationale and references. |
|
01/12/2022 |
Annual review, no change to policy intent. Updating rationale and references. |
|
01/13/2021 |
Annual review, reformatting for clarity, updating policy number. Adding verbiage regarding testing for a diagnosis of MTC. Also updating description, rationale and references. |
|
01/01/2020 |
Annual review, no change to policy intent. |
|
01/09/2019 |
Annual review, updating title for specificity and adding coverage criteria for individuals with a clinical diagnosis of MEN2. |
|
01/17/2018 |
Annual review, no change to policy intent. |
|
04/26/2017 |
Interim review to align with Avalon quarterly schedule. Updated category to Laboratory. |
|
12/29/2016 |
Annual review. No changes made to policy. |
|
04/27/2016 |
Interim review to add verbiage #5 in the policy. |
|
01/04/2016 |
Updated CPT codes. no change to intent of policy. |
|
12/14/2015 |
Interim review, adding policy language to allow coverage for "individual with apparently sporadic medullary cancer". |
|
11/04/2015 |
Annual review, no change to policy intent. Added ICD 10 coding. |
|
11/06/2014 |
Annual review, no change to policy intent. Added guidelines and coding. |
|
11/04/2013 |
Added Benefit Application and Rationale |