Influenza Vaccine - CAM 015
Description:
The Influenza virus vaccine imparts immunity against the influenza virus by stimulating production of antibodies that are specific to the disease. Patients who receive the
vaccine will be immune only to those strains of the virus from which the vaccine was prepared. Influenza viruses are recognized by the surface antigens they carry, and two
such antigens, hemagglutinin (H) and neuraminidase (N) have been identified and are used to classify the various viruses. Subtypes of these strains (H1, H2, H3, N1, N2) are
associated with influenza A virus and have been recognized to cause disease in humans. Immunity to these surface antigens increases resistance to infection and decreases the severity of the disease if infection occurs. Eventually, antigenic variation can occur, and immunity to one strain may no longer impart immunity to distantly related subtypes of the virus. Influenza B viruses also exhibit antigenic variation, and new variants of both types of viruses continue to cause widespread epidemics of respiratory disease. The below list provides available influenza vaccine at the development of this publication.
Background
The CDC states that the best way to prevent the flu is by getting a flu vaccine each year. All individuals ≥ 6 months of age should receive the flu vaccine annually.
Influenza (the flu) is a contagious respiratory illness caused by influenza viruses. It can cause mild to severe illness, and at times can lead to death. Over a period of 30 years, between 1976 and 2006, the flu has led to 3,000 up to 49,000 deaths each year and on average 226,000 hospitalizations each year. Seasonal influenza may also lead to death from other causes, such as pneumonia, an infection of the lungs that is usually caused by bacteria or viruses. Globally, pneumonia causes more deaths than any other infectious disease. The CDC estimates that the burden of illness during the 2017 – 2018 season was high, with an estimated 48.8 million people getting sick with influenza, 22.7 million people going to a health care provider, 959,000 hospitalizations, and 79,400 deaths from influenza. The number of cases of influenza‐associated illness that occurred in the 2017 – 2018 season was the highest since the 2009 H1N1 pandemic, when an estimated 60 million people were sick with influenza. Data regarding the 2018 – 2019 influenza season is not available at the timing of this publication.
Signs and symptoms of flu
People who have the flu often feel some or all of these signs and symptoms:
- Fever* or feeling feverish/chills
- Cough
- Sore throat
- Runny or stuffy nose
- Muscle or body aches
- Headaches
- Fatigue (very tired)
- Some people may have vomiting and diarrhea, though this is more common in children than adults.
*Not everyone with flu will have a fever.
Contraindications may include:
- Severe allergic reaction (anaphylaxis) to any component of the vaccine, including egg protein, or to a previous dose of any influenza vaccine.
- Concomitant aspirin therapy in children and adolescents (FluMist only).
The following warnings and precautions should be taken into account prior to administration of the influenza vaccine:
- Guillain‐Barré Syndrome — If Guillain-Barré syndrome has occurred within 6 weeks of receipt of prior influenza vaccine, the decision to give influenza vaccine should be based on careful consideration of the potential benefits and risks.
- Altered Immunocompetence — Immunocompromised persons, including individuals receiving immunosuppressive therapy, the expected immune response may not be obtained.
- Latex Allergy — The prefilled syringe tip caps for Fluarix, Fluvirin, Fluzone, and Fluzone high dose, contain natural rubber latex which may cause allergic reactions in latex‐sensitive individuals.
- Wheezing — In clinical trials, risks of hospitalization and wheezing were increased in children younger than 2 years of age who received FluMist. Children younger than 5 years of age with recurrent wheezing and persons of any age with asthma may be at increased risk of wheezing following the administration of FluMist.
Certain patients should not receive the nasal spray flu vaccine, including:
- Children younger than two years of age and adults older than 50.
- Pregnant women (Pregnancy Category B).
- People with a medical condition that places them at higher risk for complications from influenza, including those with chronic heart or lung disease, such as asthma or reactive airways disease; people with medical conditions such as diabetes or kidney failure; or people with illnesses that weaken the immune system, or who take medications that can weaken the immune system.
- Any patient with asthma.
- Children 2 – 4 years of age who had wheezing in the past 12 months. Children or adolescents 2 through 17 years of age currently taking aspirin or aspirin-containing medication. Children or adolescents should not be given aspirin for 4 weeks after getting FluMist or FluMist Quadrivalent unless your health care provider tells you otherwise.
- Patients who have had Guillain‐Barré syndrome (GBS), a rare disorder of the nervous system, with any prior influenza vaccinations.
- People who have a severe allergy to egg proteins, chicken proteins, gentamicin, gelatin, arginine, or who are allergic to any of the nasal spray vaccine components.
Common side effects for the flu shot may include mild fever, body aches, and fatigue for a few days after the vaccine, and soreness at the injection site.
The most common side effects seen with administration of the FluMist intranasal vaccine include runny nose or nasal congestion, headache, sore throat, tiredness/weakness, muscle aches, cough, fever, and chills.
The most common side effects observed with the administration of Fluzone Intradermal vaccine include injection‐site reactions, erythema, induration, swelling, pain, and pruritus. Erythema, induration, swelling, and pruritus occurred more frequently following Fluzone Intradermal than Fluzone.
The Advisory Committee on Immunization Practices (ACIP) voted to recommend a preference for using the live attenuated influenza virus nasal spray instead of the flu shot in healthy children 2 – 8 years of age when it is immediately available. This is based on a review of studies that suggests the nasal spray can provide better protection than the flu shot in this age group. If the nasal spray is not immediately available the flu shot should be given so that opportunities to vaccinate children are not missed.
Special Consideration Regarding Egg Allergy:
People with egg allergies can receive an licensed, recommended age‐appropriate influenza vaccine (IIV, RIV4, or LAIV4) that is otherwise appropriate. People who have a history of severe egg allergy (those who have had any symptom other than hives after exposure to egg) should be vaccinated in a medical setting, supervised by a health care provider who is able to recognize and manage severe allergic reactions. For those persons 18 – 49 years of age with an egg allergy of any severity, Flublok is available. It is an egg‐free vaccine option for those who could not previously receive a flu shot due to an egg allergy. It is the only licensed flu vaccine that does not use eggs in the manufacturing process.
Policy:
Standard or preservative-free injectable influenza vaccine is a MEDICALLY NECESSARY preventive service for members when influenza immunization is recommended by the Centers for Disease Control (CDC) Advisory Committee. The current CDC recommendation is that all persons aged 6 months or older receive influenza vaccine.
NOTE: Some plans exclude preventive services. Please check benefit plan descriptions for details on coverage.
Intranasally administered influenza vaccine is considered a MEDICALLY NECESSARY alternative to injectable influenza vaccine for immunocompetent healthy persons 2 to 49 years of age as recommended by the CDC's Advisory Committee on Immunization Practices (ACIP).
Rationale:
Timing of Vaccination
- In general, health care providers should begin offering vaccination soon after vaccine becomes available, and, if possible, by October.
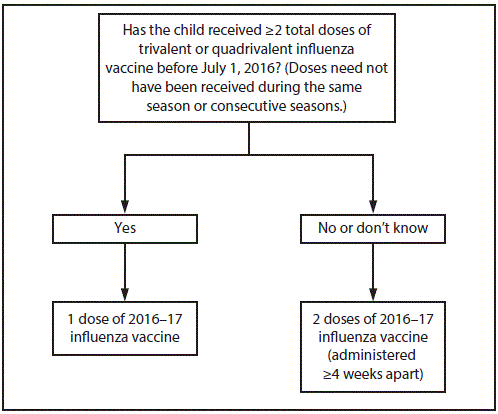
- All children ages 6 months to 8 years who are recommended for two doses should receive their first dose as soon as possible after vaccine becomes available. These children should receive the second dose ≥ four weeks later.
Persons at Risk for Medical Complications Due to Influenza
Vaccination to prevent influenza is particularly important for persons who are at increased risk for severe complications from influenza, or at higher risk for influenza-related outpatient, emergency department or hospital visits. When vaccine supply is limited, vaccination efforts should focus on delivering vaccination to the following persons (no hierarchy is implied by order of listing):
- All children aged 6 months through 59 months
- All persons aged ≥ 50 years
- Adults and children who have chronic pulmonary (including asthma) or cardiovascular (except isolated hypertension), renal, hepatic, neurological, hematologic or metabolic disorders (including diabetes mellitus)
- Persons who have immunosuppression (including immunosuppression caused by medications or by HIV infection)
- Women who are or will be pregnant during the influenza season
- Children and adolescents (aged 6 months – 18 years) who are receiving long-term aspirin therapy and who might be at risk for experiencing Reye’s syndrome after influenza virus infection
- Residents of nursing homes and other long-term care facilities
- American Indians/Alaska Natives
- Persons who are morbidly obese (BMI ≥ 40)
Persons Who Live With or Care for Persons at Higher Risk for Influenza-Related Complications
All persons aged ≥ 6 months should be vaccinated annually. Continued emphasis should be placed on vaccination of persons who live with or care for persons at higher risk for influenza-related complications. When vaccine supply is limited, vaccination efforts should focus on delivering vaccination to persons at higher risk for influenza-related complications listed above, as well as these persons:
- Health care personnel (HCP)
- Household contacts (including children) and caregivers of children aged ≤ 59 months (i.e., aged < 5 years) and adults aged ≥ 50 years, with particular emphasis on vaccinating contacts of children aged < 6 months
- Household contacts (including children) and caregivers of persons with medical conditions that put them at higher risk for severe complications from influenza
HCP and persons who are contacts of persons in these groups and who are not contacts of severely immunocompromised persons (those living in a protective environment) may receive any influenza vaccine that is otherwise indicated. People who care for the severely immunocompromised should receive either IIV or RIV3.
Influenza Vaccination for Pregnant Women
- Women who are or will be pregnant during influenza season should receive IIV. Live attenuated influenza vaccine (LAIV) is not recommended for use during pregnancy.
- Postpartum women can receive either LAIV or IIV.
- Pregnant and postpartum women do not need to avoid contact with persons recently vaccinated with LAIV.
Concurrent Administration of Influenza Vaccine With Other Vaccines
- Inactivated vaccines do not interfere with the immune response to other inactivated vaccines or to live vaccines.
- Inactivated or live vaccines can be administered simultaneously with LAIV.
- However, after administration of a live vaccine, at least 4 weeks should pass before another live vaccine is administered.
Concurrent Administration of Influenza Vaccine With Other Vaccines
- Inactivated vaccines do not interfere with the immune response to other inactivated vaccines or to live vaccines.
- Inactivated or live vaccines can be administered simultaneously with LAIV.
- However, after administration of a live vaccine, at least 4 weeks should pass before another live vaccine is administered.
|
TABLE 1. Influenza Vaccines — United States, 2016 – 2017 Influenza Season* |
|||||||
|---|---|---|---|---|---|---|---|
|
Trade name |
Manufacturer |
Presentation |
Age indications
|
Mercury (from thimerosal) μg/0.5 mL |
Latex |
Route |
|
|
Inactivated influenza vaccine, quadrivalent (IIV4), standard dose† |
|||||||
|
Fluarix Quadrivalent |
GlaxoSmithKline |
0.5 mL single-dose prefilled syringe |
≥ 3 yrs. |
NR |
No |
IM§ |
|
|
FluLaval Quadrivalent |
ID Biomedical Corporation of Quebec (distributed by GlaxoSmithKline) |
5.0 mL multi-dose vial |
≥ 3 yrs. |
NR |
No |
IM |
|
|
|
5.0 mL multi-dose vial |
≥ 3 yrs. |
<25 |
|
|
||
|
Fluzone Quadrivalent
|
Sanofi Pasteur
|
0.25 mL single-dose prefilled syringe |
6 through 35 mos. |
NR |
No |
IM |
|
|
0.5 mL single-dose prefilled syringe |
≥3 6 mos. |
NR |
No |
IM |
|||
|
0.5 mL single-dose syringe |
≥ 36 mos. |
NR |
No |
IM |
|||
|
5.0 mL multi-dose vial |
≥6 mos. |
25 |
No |
IM |
|||
|
Fluzone Intradermal Quadrivalent¶ |
Sanofi Pasteur |
0.1 mL single-dose prefilled microinjection system |
18 through 64 yrs |
NR |
No |
ID** |
|
|
Inactivated influenza vaccine, quadrivalent, cell culture-based (ccIIV4), standard dose† |
|||||||
|
Flucelvax Quadrivalent |
Seqirus |
0.5 mL single-dose prefilled syringe |
≥ 4 yrs |
NR |
No |
IM |
|
|
Inactivated Influenza Vaccine, trivalent (IIV3), standard dose† |
|||||||
|
Afluria
|
Seqirus
|
0.5 mL single-dose prefilled syringe |
≥ 9 yrs†† |
NR |
No |
IM |
|
|
5.0 mL multi-dose vial |
≥ 9 yrs†† (needle and syringe) 18 through 64 years (jet injector) |
24.5 |
No |
IM |
|||
|
Fluvirin
|
Seqirus
|
0.5 mL single-dose prefilled syringe |
≥ 4 yrs |
≤ 1 |
Yes§§ |
IM |
|
|
5.0 mL multi-dose vial |
≥ 4 yrs |
25 |
No |
IM |
|||
|
Adjuvanted Inactivated Influenza Vaccine, trivalent (aIIV3), standard dose† |
|||||||
|
Fluad |
Seqirus |
0.5 mL single-dose prefilled syringe |
≥ 65 yrs |
NR |
Yes§§ |
IM |
|
|
Inactivated Influenza Vaccine, trivalent (IIV3), high dose |
|||||||
|
Fluzone High-Dose |
Sanofi Pasteur |
0.5 mL single-dose prefilled syringe |
≥ 65 yrs |
NR |
No |
IM |
|
|
Recombinant Influenza Vaccine, trivalent (RIV3)*** |
|||||||
|
Flublok |
Protein Sciences |
0.5 mL single-dose vial |
≥ 18 yrs |
NR |
No |
IM | |
|
Live attenuated influenza vaccine, quadrivalent (LAIV4)††† |
|||||||
|
FluMist Quadrivalent |
MedImmune |
0.2 mL single-dose prefilled intranasal sprayer |
2 through 49 yrs |
NR |
No |
NAS | |
|
|||||||
TABLE 2. Contraindications and precautions to the use of influenza vaccines — United States, 2016 – 2017 Influenza Season*
| Vaccine | Contraindications | Precautions |
|---|---|---|
| IIV | History of severe allergic reaction to any component of the vaccine† or after previous dose of any influenza vaccine | Moderate to severe illness with or without fever History of Guillain-Barré syndrome within 6 weeks of receipt of influenza vaccine |
| RIV | History of severe allergic reaction to any component of the vaccine | Moderate to severe illness with or without fever History of Guillain-Barré syndrome within 6 weeks of receipt of influenza vaccine |
| LAIV | For the 2016–17 season, ACIP recommends that LAIV not be used. Content below is provided for information. | |
| History of severe allergic reaction to any component of the vaccine† or after a previous dose of any influenza vaccine Concomitant aspirin or salicylate-containing therapy in children and adolescents Children aged 2 through 4 years who have received a diagnosis of asthma or whose parents or caregivers report that a health care provider has told them during the preceding 12 months that their child had wheezing or asthma or whose medical record indicates a wheezing episode has occurred during the preceding 12 months Children and adults who have immunosuppression (including immunosuppression caused by medications or by HIV) Close contacts and caregivers of severely immunosuppressed persons who require a protected environment Pregnancy Receipt of influenza antiviral medication within the previous 48 hours |
Moderate to severe illness with or without fever History of Guillain-Barré syndrome within 6 weeks of receipt of influenza vaccine Asthma in persons aged ≥ 5 years Other underlying medical conditions that might predispose to complications after wild-type influenza infection (e.g., chronic pulmonary, cardiovascular [except isolated hypertension], renal, hepatic, neurologic, hematologic or metabolic disorders (including diabetes mellitus) |
|
Abbreviations: ACIP = Advisory Committee on Immunization Practices; IIV = Inactivated Influenza Vaccine; LAIV = Live-Attenuated Influenza Vaccine; RIV = Recombinant Influenza Vaccine.
* Immunization providers should check Food and Drug Administration-approved prescribing information for 2016 – 2017 influenza vaccines for the most complete and updated information, including (but not limited to) indications, contraindications and precautions. Package inserts for US-licensed vaccines are available at http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm.
† History of severe allergic reaction (e.g., anaphylaxis) to egg is a labeled contraindication to the use of IIV and LAIV. However, ACIP recommends that any licensed, recommended and appropriate IIV or RIV may be administered to persons with egg allergy of any severity (see Influenza Vaccination of Persons with a History of Egg Allergy).
FIGURE. Influenza vaccine dosing algorithm for children aged 6 months through 8 years — Advisory Committee on Immunization Practices, United States, 2016 – 2017 Influenza Season
References:
- Bauchner H. Journal Watch Pediatrics and Adolescents. Flu Shots or Intranasal Flu Vaccine for Children? Reviewed Oct 2008.
- Huffman G. American Family Practice. Effectiveness of Intranasal Influenza Vaccine. January 15, 2000.
- Nichol K, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults. A randomized controlled trial. JAMA July 14, 1999;282: 137-44.
- Smith NM, Bresee JS, Shay DK, et al. Advisory Committee of Immunization Practices. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practice (ACIP). MMWR Recomm Rep. 2006;55 (RR-10):1-42.
- Loeb M. Community acquired pneumonia. In: BMJ Clinical Evidence. London, UK: BMJ Publishing Group; April 2007.
- U.S. Food and Drug Administration (FDA). FDA approves nasal influenza vaccine for use in younger children. FDA News. Rockville, MD: FDA; September 19, 2007.
- U.S. Food and Drug Administration (FDA). Additional influenza vaccine approved for upcoming season. Approval increases number of available doses to record level. FDA News. Rockville, MD: FDA; September 28, 2007.
- Lewis EN, Friffin MR, Szilagy PG, et al. Childhood influenza. Number need to vaccinate to prevent 1 hospitalization or outpatient visit. Pediatrics. 2007;120(3): 467-472.
- Treanor JD. Influenza-The goal of control. N Engl J Med. 2007; 357(14):1439-1441.
- Centers of Disease Control and Prevention (CDC). Division of Media Relations. CDC's advisory committee recommends influenza vaccination for children 6 months through 18 years of age. Press Release. Atlanta, GA; CDC; February 27, 2008.
- Fiore AE, Shay DK, Broder K, et al. Centers for Disease Control and Prevention (CDC): Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). 2008. MMWR Recomm Rep. 2008;57 (RR-7):1-60.
- U.S. Food and Drug Administration (FDA). FDA Approve 2008-2009 Flu Vaccines. FDA News. Rockville, MD: FDA ; August 5, 2008.
- http://www.cdc.gov/flu/professionals/acip/2013-summary-recommendations.htms
- http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6430a.htm
- CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices [ACIP]. https://www.cdc.gov/flu/professionals/acip/. Accessed August 2021.
- Clinical Pharmacology [database online]. Tampa, FL: Gold Standard, Inc.; 2021. URL: http://www.clinicalpharmacology.com Accessed August 2021.
- Hurwitz ES, Schonberger LB, Nelson DB, Holman RC. Guillain‐Barré syndrome and the 1978‐1979 influenza vaccine. N Engl J Med 1981;304:1557‐1561.
- Centers for Disease Control and Prevention. (2021). Influenza (Flu). URL: https://www.cdc.gov/flu/index.htm Accessed August 2021
Coding Section
| Codes | Number | Description |
| CPT | 90460 | Immunization administration through 18 years of age via any route of administration, with counseling by physician or other qualified health care professional; first or only component of each vaccine or toxoid administered |
| 90461 |
Immunization administration through 18 years of age via any route of administration, with counseling by physician or other qualified health care professional; each additional vaccine or toxoid component administered (List separately in addition to code for primary procedure) |
|
| 90471 | Immunization administration (includes percutaneous, intradermal, subcutaneous, or intramuscular injections); one vaccine (single or combination vaccine/toxoid) | |
| 90472 | Immunization administration (includes percutaneous, intradermal, subcutaneous, or intramuscular injections); each additional vaccine (single or combination vaccine/toxoid) (List separately in addition to code for primary procedure) | |
| 90473 | Immunization administration by intranasal or oral route; one vaccine (single or combination vaccine/toxoid) | |
| 90474 | Immunization administration by intranasal or oral route; each additional vaccine (single or combination vaccine/toxoid) (List separately in addition to code for primary procedure) | |
| 90630 | Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, for intradermal use | |
| 90653 | Influenza vaccine, inactivated (IIV), subunit, adjuvanted, for intramuscular use | |
| 90654 | Influenza virus vaccine, trivalent (IIV3), split virus, preservative-free, for intradermal use | |
| 90655 | Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, when administered to children 6 – 35 months of age, for intramuscular use | |
| 90655 (effective 1/1/2017) | Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, 0.25 mL dosage, for intramuscular use | |
| 90656 | Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, when administered to individuals 3 years and older, for intramuscular use | |
| 90656 (effective 8/1/2016) | Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, 0.5 mL dosage, for inramuscular use | |
| 90657 | Influenza virus vaccine, trivalent (IIV3), split virus, when administered to children 6-35 months of age, for intramuscular use | |
| 90657 (effective 8/1/2016) | Influenza virus vaccine, trivalent (IIV3), split virus, 0.25 mL dosage, for intramuscular use | |
| 90658 | Influenza virus vaccine, trivalent (IIV3), split virus, when administered to individuals 3 years of age and older, for intramuscular use | |
| 90658 (effective 1/1/2017) | Influenza virus vaccine, trivalent (IIV3), split virus, 0.5 mL dosage, for intramuscular use | |
| 90660 | Influenza virus vaccine, trivalent, live (LAIV3), for intranasal use | |
| 90661 | Influenza virus vaccine (ccIIV3), derived from cell cultures, subunit, preservative and antibiotic free, for intramuscular use | |
| 90661 (effective 8/1/2016) | Influenza virus vaccine, trivalent (ccIIV3), derived from cell cultures, subunit, preservation and antibiotic free, 0.5 mL dosage, for intramuscular use | |
| 90662 | Influenza virus vaccine (IIV), split virus, preservative free, enhanced immunogenicity via increased antigen content, for intramuscular use | |
| 90664 | Influenza virus vaccine, live (LAIV), pandemic formulation, for intranasal us | |
| 90666 | Influenza virus vaccine (IIV), pandemic formulation, split virus, preservative free, for intramuscular use | |
| 90667 | Influenza virus vaccine (IIV), pandemic formulation, split virus, adjuvanted, for intramuscular use | |
| 90668 | Influenza virus vaccine (IIV), pandemic formulation, split virus, for intramuscular use | |
| 90672 | Influenza virus vaccine, quadrivalent, live (LAIV4), for intranasal use | |
| 90673 | Influenza virus vaccine, trivalent (RIV3), derived from recombinant DNA (RIV3), hemagglutinin (HA) protein only, preservative and antibiotic free, for intramuscular use | |
| 90674 (effective 8/1/2016) | Influenza virus vaccine, quadrivalent (ccIIV4), derived from cell cultures, subnit, preservative and antibiotic free, 0.5 mL dosage, for intramuscular use | |
| 90682 (effective 1/01/2018) | INFLUENZA VIRUS VACCINE, QUADRIVALENT (RIV4), DERIVED FROM RECOMBINANT DNA, HEMAGGLUTININ (HA) PROTEIN ONLY, PRESERVATIVE AND ANTIBIOTIC FREE, FOR INTRAMUSCULAR USE | |
| 90685 | Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, when administered to children 6 – 35 months of age, for intramuscular use | |
| 90685 (effective 8/1/2016) | Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, 0.25 mL dosage, for intramuscular use | |
| 90686 | Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, when administered to individuals 3 years of age and older, for intramuscular use | |
| 90686 (effective 8/1/2016) | Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, 0.5 mL dosage, for intramuscular use | |
| 90687 | Influenza virus vaccine, quadrivalent (IIV4), split virus, when administered to children 6 – 35 months of age, for intramuscular use | |
| 90687 (effective 8/1/2016) | Influenza virus vaccine, quadrivalent (IIV4), split virus, 0.25 mL dosage, for intramuscular use | |
| 90688 | Influenza virus vaccine, quadrivalent (IIV4), split virus, when administered to individuals 3 years of age and older, for intramuscular use | |
| 90688 (effective 8/1/2016) | Influenza virus vaccine, quadrivalent (IIV4), split virus, 0.5 mL dosage, for intramuscular use | |
| 90694 | INFLUENZA VIRUS VACCINE, QUADRIVALENT (AIIV4), INACTIVATED, ADJUVANTED, PRESERVATIVE FREE, 0.5 ML DOSAGE, FOR INTRAMUSCULAR USE | |
| 90756 (effective 1/1//2018) | Influenza virus vaccine, quadrivalent (ccIIV4), derived from cell cultures, subunit, antibiotic free, 0.5mL dosage, for intramuscular use | |
| HCPCS | G0008 | Administration of influenza virus vaccine |
| G0009 | Administration of pneumococcal vaccine | |
| G0010 | Administration of hepatitis B vaccine | |
| Q2035 | Influenza virus vaccine, split virus, when administered to individuals 3 years of age and older, for intramuscular use (AFLURIA) | |
| Q2036 | Influenza virus vaccine, split virus, when administered to individuals 3 years of age and older, for intramuscular use (FLULAVAL) | |
| Q2037 | Influenza virus vaccine, split virus, when administered to individuals 3 years of age and older, for intramuscular use (FLUVIRIN) | |
| Q2038 | Influenza virus vaccine, split virus, when administered to individuals 3 years of age and older, for intramuscular use (Fluzone) | |
| Q2039 | Influenza virus vaccine, split virus, when administered to individuals 3 years of age and older, for intramuscular use (not otherwise specified) |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, Blue Cross Blue Shield Association technology assessment program (TEC) and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2014 Forward
| 09/01/2022 | Annual review, no change to policy intent. Updating description and references. Adding background. |
|
09/01/2021 |
Annual review, no change to policy intent. |
|
09/01/2020 |
Annual review, no change to policy intent. |
|
06/08/2020 |
Correcting typo in history box dated 5/28/2020. Added code 90694 to coding section. No other changes made. |
|
05/28/2020 |
Adding code 90967 to coding section. No other changes made. |
|
09/01/2019 |
Annual review, no change to policy intent. |
|
09/05/2018 |
Annual review, no change to policy intent. |
|
02/07/2018 |
Correction of date in coding section. No other changes made. |
|
08/24/2017 |
Adding Code 90756 to coding section. No change to policy intent. |
|
03/01/2017 |
Updated CPT coding. |
|
09/19/2016 |
Updated CPT coding effective date. Updated rationale tables. |
|
08/03/2016 |
Updated CPT coding in policy. |
|
08/31/2015 |
Annual review, no change to policy intent. Adding coding and updating table 1 to current ACIP recommendations. |
|
08/18/2015 |
Changed review to September, as the 2015/2016 CDC recommendations have not been released yet. |
|
08/11/2014 |
Annual review, no changes made. |