Prescription Medication and Illicit Drug Testing in the Outpatient Setting - CAM 140
Description/Background
Abuse of both prescription and illicit drugs is extremely common. Drugs of abuse (DOA) may be defined as “a drug, chemical, or plant product that is known to be misused for recreational purposes,” which can include drugs such as pain relievers that have legitimate prescriptions. Drug tests may be performed for a variety of reasons, such as compliance with treatment program or medical regimen. Numerous biological substances, such as blood, hair, or saliva may be tested, but urine is the most commonly tested biological substance in drug tests (Hoffman, 2022).
This policy addresses clinical toxicology in the outpatient setting and does not address forensic testing or therapeutic drug monitoring (TDM). Forensic drug testing is used for legal proceedings and requires secondary confirmatory testing (Jones, 2016). TDM “involves sa4mpling of plasma or serum drug levels to determine optimal drug dosing” (Eaton & Lyman, 2022).
Regulatory Status
A multitude of urine drug tests are available and approved by the FDA. Additionally, many labs have developed specific tests that they must validate and perform in house. These laboratory-developed tests (LDTs) are regulated by the Centers for Medicare & Medicaid Services (CMS) as high-complexity tests under the Clinical Laboratory Improvement Amendments of 1988 (CLIA ’88). As an LDT, the U.S. Food and Drug Administration has not approved or cleared this test; however, FDA clearance or approval is not currently required for clinical use.
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
This policy concerns only coverage criteria and does not describe or define the legal responsibility of providers. Providers should refer to state and federal laws for such guidance.
This policy does not address the use of drug testing in the following circumstances:
- State, federally regulated, and legally mandated drug testing (i.e., court-ordered drug screening, forensic examinations).
- Non-forensic testing for commercial driver’s licensing or any other job-related testing (i.e., as a prerequisite for employment or as a means for continuation of employment).
- As a component of routine physical/medical examination.
- As a component of care rendered in an urgent/emergency situation.
- As a routine component of a behavioral health assessment.
PRESUMPTIVE DRUG SCREENING USING URINE SAMPLES
- Presumptive drug screening using urine samples (qualitative, semi-quantitative or quantitative) is considered MEDICALLY NECESSARY in any of the following situations:
- To assess an individual being treated for chronic, non-cancer pain when clinical evaluation of the individual (history/signs/symptoms) suggests the use of non-prescribed medications or illegal substances:
- Prior to initiating chronic opioid pain therapy in chronic non-cancer pain to determine if the individual has been exposed to controlled substances or potentially confounding illicit drugs.
- To verify an individual’s compliance with treatment or identify undisclosed drug abuse as part of routine monitoring for individuals who are receiving treatment for non-cancer chronic pain with prescription opioid pain medication. The random testing interval and drugs selected for testing should be based on the individual’s history, condition, and treatment, as documented in the medical record.
- Monitoring of low risk (as defined by a risk assessment tool) individuals on chronic opioid therapy, up to one time per year after initiation of therapy.
- Monitoring of moderate risk (as defined by a risk assessment tool) individuals on chronic opioid therapy, up to two times per year after initiation of therapy.
- Monitoring of high risk (as defined by a risk assessment tool) individuals on chronic opioid therapy, up to four times per year after initiation of therapy.
- For individuals with aberrant behavior (lost prescriptions, multiple requests for early refills, and opioids from multiple providers, unauthorized dose escalation, apparent intoxication, etc.), testing at the time of visit meets coverage criteria.
- In pregnant individuals at high-risk for substance abuse in whom the suspicion of drug use exists based on the answers to substance abuse screening questions or as indicated by information from the prescription drug monitoring program (PDMP), as documented in the medical record.
- In newborns when there is a history of maternal substance abuse or agitated/altered mental status in the birthing parent.
- In candidates for organ transplant who have a history of substance abuse (to demonstrate abstinence prior to transplant).
- In individuals with a suspicion of or a diagnosis of mental illness (e.g., anxiety disorders, schizophrenia, major depressive disorder, mood disorders, suicidal ideations, substance abuse disorder).
- In individuals with attention-deficit hyperactivity and disruptive behavior disorders.
- In cancer patients on opioid pain medication.
- In individuals with epilepsy.
- For the management and compliance monitoring of an individual under treatment for substance abuse or dependence at the following frequency (after baseline at initial evaluation) and must be documented in the patient’s medical record:
- For patients with 0 to 90 consecutive days of abstinence, random qualitative drug testing at a frequency of 1 to 2 per week.
- To assess an individual being treated for chronic, non-cancer pain when clinical evaluation of the individual (history/signs/symptoms) suggests the use of non-prescribed medications or illegal substances:
-
-
- For patients with > 90 consecutive days of abstinence, random qualitative drug testing at a frequency of 1 to 3 per month.
- In individuals where substance abuse is in the differential diagnosis of the presenting conditions.
-
DEFINITIVE DRUG TESTING
- Confirmatory/definitive qualitative or quantitative drug testing (up to seven drug classes) is considered MEDICALLY NECESSARY when laboratory-based definitive drug testing is specifically requested, the rationale is documented by the patient’s treating physician, and any of the following conditions are met:
- The result of the presumptive drug screen is different than that suggested by the patient’s medical history, their clinical presentation, or patient’s own statement (e.g., test was negative for prescribed medications, test was positive for prescription drug with abuse potential which was not prescribed, test was positive for an illegal drug).
- For diagnosing and monitoring individuals with substance use disorder or dependence, when accurate and reliable results are necessary for treatment decisions:
- Individuals with 0 to 30 consecutive days of abstinence, random definitive drug testing at a frequency not to exceed 1 per week.
- Individuals with 31 to 90 consecutive days of abstinence, random definitive drug testing at a frequency of 1 to 3 per month. No more than 3 definitive drug tests in one month will be allowed.
- Individuals with greater than 90 consecutive days of abstinence, definitive drug testing at a frequency of 1 to 3 every three months. No more than 3 definitive drug tests in a 3-month period will be allowed.
- For monitoring of individuals on opioid therapy (to ensure adherence to the therapeutic plan, for treatment planning, and for detection of other, non-prescribed opioids).
- A presumptive test does not exist or does not adequately detect the specific drug or metabolite to be tested (e.g., specific drugs within the amphetamine, barbiturate, benzodiazepine, tricyclic antidepressants, and opiate/opioid drug classes, as well as synthetic/analog or “designer” drugs).
- To definitively identify specific drugs in a large family of drugs.
- To identify drugs when a definitive concentration of a drug is needed to guide management.
- When laboratory-based definitive drug testing is requested for larger than seven drug classes panels, confirmatory/definitive qualitative or quantitative drug testing is considered NOT MEDICALLY NECESSARY.
- Confirmatory/definitive qualitative or quantitative or presumptive (qualitative, semi-quantitative or quantitative) drug testing using proprietary tests (e.g., RiskViewRx Plus) is considered NOT MEDICALLY NECESSARY.
SPECIMEN VALIDITY TESTING
- Specimen validity testing (e.g., urine specific gravity, urine creatinine, pH, urine oxidant level, genetic identity testing [e.g., NextGen Precision™ Testing]) is considered NOT MEDICALLY NECESSARY.
NOTES:
Documentation Requirements
The patient's medical record must contain documentation that fully supports the medical necessity for drug testing. This documentation includes, but is not limited to, relevant medical history, physical examination, and results of pertinent diagnostic tests or procedures.
- Reimbursement
- The following IS reimbursed (see complete Coverage Criteria in Letters A and B, Section III above) for:
- Presumptive drug screening based upon appropriate clinical criteria (qualitative, semi-quantitative or quantitative);
- Definitive drug testing (qualitative or quantitative) for up to seven drug classes when the presumptive drug screening meets one of the following criteria:
- The test was negative for prescribed medications, or
- Positive for a prescription drug with abuse potential which was not prescribed, or
- Positive for an illegal drug, or
- A presumptive test does not exist or does not adequately detect the specific drug or metabolite to be tested.
- Blood specimens in patients with anuric chronic renal failure.
- The following is considered NOT MEDICALLY NECESSARY:
- Any AMA definitive drug class codes
- Same-day testing of the same drug or metabolites from two different samples (e.g. both a blood and a urine specimen) by either presumptive or definitive analyses
- Blanket orders or routine standing orders for all patients in the physician’s practice
- Only urine or oral fluid specimens will be covered except blood specimen will be covered for patients with anuric chronic renal failure.
- Confirmatory/definitive testing should be supported by documentation of rationale in the patient’s medical record.
- More than one presumptive test result per patient per date of service regardless of the number of billing providers is considered NOT MEDICALLY NECESSARY:
- It is not reasonable or necessary for a provider to perform qualitative point-of-care testing and also order presumptive testing from a reference laboratory on the same specimen.
- It is not reasonable or necessary for a provider to perform presumptive immunoassay testing and also order presumptive immunoassay testing from a reference laboratory with or without reflex testing on the same specimen.
Table of Terminology
|
Term |
Definition |
|
6-AM |
6-acetylmorphine |
|
6-MAM |
6-monoacetylmorphine |
|
AACAP |
American Academy of Child and Adolescent Psychiatry |
|
AACC |
American Association for Clinical Chemistry |
|
AAFP |
American Academy of Family Physicians |
|
AAN |
American Academy of Neurology |
|
AAPM |
American Academy of Pain Medicine |
|
AATOD |
American Association for The Treatment of Opioid Dependence Inc. |
|
ACOEM |
American College of Occupational and Environmental Medicine |
|
ACOG |
American College of Obstetricians and Gynecologists |
|
ADAC |
Anxiety Disorders Association of Canada |
|
ADHD |
Attention-deficit/hyperactivity disorder |
|
AMA |
American Medical Association |
|
AMDG |
Agency Medical Directors' Group |
|
APA |
American Psychiatric Association |
|
ASAM |
American Society of Addiction Medicine |
|
ASIPP |
American Society of Interventional Pain Physicians |
|
AUDIT-C |
Alcohol use disorders identification test-consumption |
|
BD |
Bipolar disorder |
|
CDC |
Centers for Disease Control and Prevention |
|
CLIA ’88 |
Clinical Laboratory Improvement Amendments Of 1988 |
|
CMS |
Centers For Medicare & Medicaid Services |
|
COT |
Chronic opioid treatment |
|
CPS |
Canadian Paediatric Society |
|
CYP2D6 |
Cytochrome P450 2D6 |
|
DNA |
Deoxyribonucleic acid |
|
DOA |
Drugs of abuse |
|
DOD |
Department Of Defense |
|
DVA |
Department of Veterans Affairs |
|
EDDP |
2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine |
|
EIAs |
Enzyme immunoassays |
|
EMIT |
Enzyme multiplied immunoassay technology |
|
FDA |
Food and Drug Administration |
|
FSMB |
Federation Of State Medical Boards |
|
GABA |
Gamma aminobutyric acid |
|
GAD |
Generalized anxiety disorder |
|
GC |
Gas chromatography |
|
GHB |
Gamma-hydroxybutyrate |
|
HHS |
Department Of Health and Human Services |
|
HIV |
Human immunodeficiency virus |
|
JA |
Joint arthroplasty |
|
LC |
Liquid chromatography |
|
LCD |
Local coverage determinations |
|
LDTs |
Laboratory-developed tests |
|
LSD |
Lysergic acid diethylamide |
|
MDMA |
3,4-methylenedioxymethamphetamine |
|
MS |
Mass spectrometry |
|
MTF |
Monitoring the future |
|
NACB |
National Academy of Clinical Biochemistry |
|
NICE |
National Institute for Health and Care Excellence |
|
NIDA |
National Institute of Drug Abuse |
|
NMDA |
N-methyl-d-aspartic acid |
|
NOUGG |
National Opioid Use Guideline Group |
|
NSDUH |
National Survey on Drug Use and Health |
|
OASAS |
Office of Addiction Services and Supports |
|
OTPs |
Opioid treatment programs |
|
OUD |
Opioid use disorder |
|
PCP |
Phencyclidine |
|
PDMP |
Prescription drug monitoring program |
|
POC |
Point-of-care |
|
SAD |
Social anxiety disorder |
|
SAMHSA |
Substance Abuse and Mental Health Services Administration |
|
SASQ |
Single item alcohol screening questionnaire |
|
SOAPP |
Screener and opioid assessment for patients with pain |
|
SOGC |
Society Of Obstetricians and Gynaecologists of Canada |
|
SUD |
Substance use disorder |
|
TCAs |
Tricyclic antidepressants |
|
TDM |
Therapeutic drug monitoring |
|
THC |
Tetrahydrocannabinol |
|
TLC |
Thin layer chromatography |
|
UDM |
Urine drug monitoring |
|
UDS |
Urine drug screening |
|
UDT |
Urine drug testing |
|
UMHS |
University of Michigan Health System |
|
VA/DOD |
Department Of Veterans Affairs/Department of Defense |
|
WFSBP |
World Federation of Societies of Biological Psychiatry |
|
WHO |
World Health Organization |
Rationale
According to the National Center for Drug Abuse Statistics, as many as 31.9 million Americans 12 or older used an illicit drug in the last 30 days, which corresponds to 11.7% of Americans overall and 39% for young adults from 18 to 25. 10.1 million misused opioids in the previous year, with 9.7 million misusing prescription pain relievers. Approximately 9.5 million adults had a concurrent mental illness and substance abuse disorder in the previous year (National Center for Drug Abuse Statistics, 2022). A drug of abuse (DOA) may be defined as “a drug, chemical, or plant product that is known to be misused for recreational purposes,” which can include drugs, such as pain relievers, that have legitimate prescriptions. Drug testing may be performed for several reasons. For example, patients in areas including pain management, substance abuse treatment, and psychiatric treatment have a higher propensity for substance abuse and must be monitored as such (Hoffman, 2022).
DOA screening varies in composition between countries. In the U.S., typical DOA screening tests encompass amphetamine, cocaine, marijuana/tetrahydrocannabinol (THC), opioids, and phencyclidine (PCP) as included in the United States’ Drug-Free Workplace Act of 1988; these DOA are often referred as the SAMHSA 5, named after the Substance Abuse and Mental Health Services Administration (Hoffman, 2022; Phan et al., 2012). Although the drug trends have changed dramatically since 1988, these five have remained on the basic drug screen used across the U.S. The U.S. Department of Defense (DOD) removed PCP from its routine screening but added benzodiazepines, amphetamine derivatives, common barbiturates, synthetic and semisynthetic opioids, lysergic acid diethylamide (LSD), and synthetic cannabinoids. Other countries or regions, such as Australia and the European Union, also include testing for benzodiazepines and wider range of opioids (Hoffman, 2022). The American Society of Addiction Medicine (ASAM) recommends drug-testing panels based on “the patient’s drug of choice, prescribed medications, and drugs commonly used in the patient’s geographic location and peer group” rather than relying on the SAMHSA 5 (ASAM, 2017).
The testing performed could be qualitative, semi-quantitative or quantitative, presumptive, or definitive. Qualitative refers to testing for the presence of a given analyte, semi-quantitative reports if the analyte is present above or below a certain threshold, and quantitative reports the exact amount of an analyte. Presumptive drug testing is used to identify use or non-use of a drug or a drug class, but this type of testing cannot distinguish between structural isomers. Definitive drug testing usually refers to a more definitive methodology, such as mass spectrometry or chromatography, because these methods can identify use or non-use of a specific drug and/or its associated metabolites. Both types of drug testing can be either quantitative or qualitative (Jannetto & Langman, 2018). The frequency of testing is usually determined by the providers; testing may be random or scheduled depending on the provider’s objectives (Becker & Starrels, 2022).
Urine drug tests are the most common method of drug testing for several reasons. Unlike blood or saliva, the window of detection of most drugs is longer in urine; moreover, urine tests are inexpensive, noninvasive, and convenient to use while still maintaining acceptable statistical validity. Salivary testing can provide a higher rate of false-negative results, especially for individuals who smoke. Urine may provide more objective assessment of drug levels compared to purely clinician evaluation or a patient self-report (Becker & Starrels, 2022). A disadvantage of urine testing is “a high risk of adulteration of the sample by the patient to avoid detection of non-compliance with the therapeutic regimen” (AACC, 2017). The table below, adapted from Hoffman (2022), summarizes urine drug testing assays for several drugs.
|
Drug |
Time Frame for Testing |
Substance Detected |
Potential False-Positives (Varies by Assay) |
|
Amphetamine |
1 – 2 days (acute exposure) 2 – 4 days (chronic exposure) |
Amphetamine |
Poor specificity due to structural similarities to many drugs, herbal supplements, and medications, including many nasal decongestants. |
|
Benzodiazepines (Note: No single assay is known to detect all benzodiazepines.) |
1 – 5 days for most benzodiazepines 2 – 30 days for diazepam |
Oxazepam (most common) Various metabolites |
Oxaprozin |
|
Cocaine |
2 days (acute exposure) 7 days (chronic exposure) |
Benzoylecgonine |
Coca tea, coca leaves |
|
GHB |
< 24 hours |
GHB |
“Endogenous neurotransmitter naturally present in minute quantities” |
|
Ketamine |
1 – 3 days |
Ketamine, norketamine |
|
|
LSD |
1 – 3 days |
2-oxo-3-hydroxy-LSD |
|
|
Marijuana (Note: Synthetic cannabinoids are not usually detected by routine urine assays.) |
1 – 3 days (acute exposure) > 1 month (chronic exposure) |
11-nor-9-carboxy-Δ9-THC |
Hemp-containing foods or hemp products (e.g., hemp soap) in rare cases |
|
Opioids (Synthetic opioids are not detected by routine opioid screening, though specific assays such as buprenorphine are available.)
|
1 – 3 days |
Morphine and all natural opioids (e.g., codeine) |
Poppy seeds (Note: The threshold for urine detection has been substantially raised to decrease the likelihood of poppy seed false-positives.) |
|
Methadone |
1 – 5 days |
Methadone EDDP |
Doxylamine |
|
PCP |
4 – 7 days |
PCP |
Dextromethorphan, diphenhydramine, doxylamine, ketamine, tramadol, venlafaxine |
Presumptive urine drug testing (UDT) typically uses an immunoassay where antibodies detect the drug or drug metabolite. This testing can be either qualitative, showing only a positive or negative finding, or semi-quantitative. Immunoassays offer fast turnaround times but can also give false-positive or false-negative results. Federal Workplace Drug Testing Programs usually use higher cutoff values to avoid false-positive results, but this can increase the likelihood of false-negatives (AACC, 2017). One study reports a false-negative rate of 28% for detecting benzodiazepines (Manchikanti et al., 2010). Another approach is to utilize orthogonal testing where an initial immunoassay is followed by a spectroscopic assay. This can be used for monitoring compliance in pain management therapy (AACC, 2017). Regardless, proper interpretation of results is imperative. Inadequate physician knowledge of interpretation can limit the efficient use of UDT (Pesce et al., 2012); in fact, a single study found that 25 of 88 (28%) of UDT results were susceptible to provider interpretation error when compared to the laboratory toxicologist’s interpretation (Chua et al., 2020).
Presumptive point-of-care (POC) testing is also available. POC tests use either a urine or saliva sample to screen for drugs in an immunoassay. Like laboratory-based immunoassays, POC testing has lower sensitivity and specificity than definitive drug tests; however, they can provide immediate results to the physician where a negative result typically rules out DOA and a positive result requires confirmatory testing (AACC, 2017). False-positive and false-negative results are even more problematic in POC testing than laboratory-based immunoassays. The clinician must be cognizant of medications — both prescribed and over-the-counter — that can trigger false-positives; for example, over-the-counter nasal inhalers can contain active ingredients that give a potential false-positive for methamphetamine. Moreover, POC testing may not be capable of detecting medications that are metabolites of parent medications (Pesce et al., 2012).
Definitive drug testing typically uses chromatographic and spectroscopic methodologies, including gas chromatography (GC) or liquid chromatography (LC) and tandem mass spectrometry (MS). According to the American Association for Clinical Chemistry (AACC), MS-based assays are traditionally considered the gold standard even though they are both more labor- and time-intensive. Whereas immunoassay-based assays usually only detect a class of compounds, MS-based assays can detect specific drugs in urine samples (AACC, 2017).
Opioids
Opioids are the standard of care for moderate to severe pain, and primarily work by stimulating the µ, δ, or κ opioid receptors in the central nervous system and throughout the body (Owusu Obeng et al., 2017). The stimulation of these receptors typically causes blocking of pain neurotransmitters such as glutamate and blocks the release of GABA, thereby producing extra dopamine. This extra dopamine also creates a pleasurable effect and possible euphoria (Trescot et al., 2008).
However, due to their mechanism of action, opioids and other pain relievers can cause addiction and are widely abused. According to the CDC, over 142 million prescriptions for opioids were written in 2020 (CDC, 2022b). Although the overall trend in annual opioid prescribing rates have been falling from the peak in 2012 of 81.3 prescriptions per 100 persons to 43.3 per 100 in the most recently reported year (2020)(CDC, 2022b), opioid abuse is still extremely widespread and considered an “epidemic” in the United States. According to the CDC, in 2019, a 4% increase in the number of age-adjusted rate of drug overdose deaths occurred, and 70.6% of all drug overdose deaths involved the use of opioids (CDC, 2022a). In 2019, a total of 70,630 drug overdose deaths occurred in the United States (CDC, 2022a). The CDC monitors the number of deaths and nonfatal overdoses of opioids in four categories (CDC, 2017):
- Natural/semi-synthetic opioids, such as morphine and oxycodone, respectively
- Methadone
- Synthetic opioids other than methadone
- Heroin
Immunoassay-based screening tests for opioids typically detect morphine, a common metabolite in natural opioids and heroin; however, synthetic opioids, such as fentanyl, methadone, and tramadol, and semisynthetic opioids, including hydrocodone and oxycodone, are not detected using routine opioid screening. These drugs are detected using a specific screening assay. Previously, poppy seed consumption triggered false-positive results, so the U.S. Substance Abuse Mental Health Services Administration (SAMHSA) raised the urine threshold for morphine from 300 ng/mL to 2000 ng/mL. Additionally, heroin can be distinguished from poppy seed exposure by testing for 6-monoacetylmorphine (6-MAM) (Hoffman, 2022). 6-MAM has a short half-life before it metabolizes to morphine; the absence of 6-MAM does not rule heroin use (Pesce et al., 2018).
Non-Opioid Medications Used in Chronic Pain Management
Other non-opioid medications can be used in chronic pain management, including antidepressants, anticonvulsants, neuroleptics, antispasmodics, and muscle relaxants. Tricyclic antidepressants (TCAs), such as nortriptyline, are used in pain management even though the analgesic mechanism is unknown. At times, TCAs may be used as adjuncts to opioid therapy to potentiate the analgesic effect of the opioid for individuals suffering from severe pain and/or diabetic neuropathy. Certain newer anticonvulsants, such as pregabalin and gabapentin, can be used as first-line agents in chronic pain treatment due to favorable side effect profiles. Neuroleptics can be used, especially for patients with psychotic symptomology, but these drugs can have undesirable long-term side effects, including akathisia and tardive dyskinesia. Pain due to muscle spasms in certain individuals may be relieved using muscle relaxants and antispasmodics, including baclofen. These non-opioid medications may be monitored for compliance similarly to their opioid counterparts in patients. The table below lists examples of common non-opioid medications that may be used for pain management (AACC, 2017).
|
Antidepressants |
Anticonvulsants |
Neuroleptics |
Antispasmodics and Muscle Relaxants |
|
Doxepin Amitriptyline Imipramine Nortriptyline Desipramine Venlafaxine Duloxetine |
Phenytoin Gabapentin Pregabalin Carbamazepine Oxcarbazepine Clonazepam |
Fluphenazine Haloperidol Chlorpromazine Perphenazine |
Baclofen Cyclobenzaprine Carisoprodol |
Benzodiazepines and Barbiturates
Due to their anxiolytic and hypnotic properties, tranquilizers, such as benzodiazepines—including Xanax, Valium, and Restoril—have an especially high rate of abuse as they are frequently prescribed for common disorders, such as anxiety and insomnia. Benzodiazepine intoxication has similar features to alcohol intoxication; severe overdose leads to respiratory depression and eventual anoxic brain damage or death (Weaver, 2015). Benzodiazepines consist of approximately 90% of tranquilizer abuse (Becker & Starrels, 2022) and consisted of approximately 30% of deaths from a pharmaceutical agent in 2010 (Jones et al., 2013). Benzodiazepines are not typically included in the standard urine screening for DOA, but the most common test for benzodiazepines identifies metabolites of 1,4-benzodiazepines like oxazepam. Benzodiazepines that do not metabolize in this manner (such as Xanax) may not be detected. Furthermore, a positive test only indicates a recent exposure to the drug indicated (Greller & Gupta, 2020). The HIV treatment efavirenz gives a false-positive result in benzodiazepine screening; in fact, one study reported that 98% of urine samples of individuals on efavirenz gave a false-positive as compared to only 2% of the control group (Blank et al., 2009). Testing for benzodiazepines is particularly important if opioids or alcohol are involved; 28% of all prescription opioid overdoses in 2015 involved benzodiazepines (Kandel et al., 2017). And, false negative results are often seen in a pain management population in patients prescribed lorazepam and clonazepam because benzodiazepine immunoassays are inadequately sensitive (Jannetto et al., 2017).
Although barbiturates, another class of sedatives, are not prescribed as much as in the past, they are still an abusable drug and have use as an anesthetic and anticonvulsant. Barbiturates are also frequently prescribed for headaches, which can lead to physical withdrawal in the form of recurrent headaches (Weaver, 2015). Similar to benzodiazepines, barbiturates can produce a hypnotic and relaxing effect, but euphoria may be a side effect depending on dose (Eskridge & Guthrie, 1997). Its harmful side effects are similar to those of benzodiazepine poisoning (e.g., respiratory depression, slowed mental state) (Greller & Gupta, 2020). The barbiturate immunoassay typically detects secobarbital; the most frequently prescribed barbiturates of phenobarbital, primidone, and butalbital are detected well by barbiturate immunoassays (Algren & Christian, 2015). POC tests, such as the Instant-View® Barbiturate Urine Test, can be used for initial screening but should have confirmatory testing for positive results. According to its package insert, besides phenobarbital, “this test is designed to detect unchanged secobarbital in the urine; however, as with some other analytical methods such as EMIT and RIA, this assay can also detect other commonly encountered barbiturates, depending on the concentration of drug present in the sample. With standard single dose of secobarbital, pentobarbital, or amobarbital, positive results may be identified from 30 hours to 76 hours (ALFA, 2019).” A positive response rate of detection is reported with minimal concentrations of 200 – 300 ng/mL, depending on the barbiturate. The Wondfo Barbiturates Urine Test is another FDA approved POC test which provides results in five minutes. This test can identify 16 drugs including barbiturates and benzodiazepines with a single testing strip (Wondfo, 2020).
Amphetamines
Stimulants, including amphetamines and drugs prescribed for attention-deficit/hyperactivity disorder (ADHD), can be abused due to their euphoric side effects (Moeller et al., 2017). Although there are many different kinds of stimulants, their primary mechanism of action is blocking the dopamine receptor or stimulating release of dopamine (Kampman, 2022). Amphetamine side effects include tachycardia, high blood pressure, and agitation; severe overdose may lead to seizures, hallucinations, or paranoia (Becker & Starrels, 2022). UDTs for amphetamines, such as the DRI® Amphetamines Assay, are immunoassays that detect amphetamine and/or methamphetamine. The DRI® Amphetamines Assay has cutoff levels of 500 ng/mL for amphetamine and 1000 ng/mL for methamphetamine with 58.0% concordance between the immunoassay and GC/MS at the 500 ng/mL cutoff. The manufacturer states, “a positive result by this assay should be confirmed by another nonimmunological method such as GC, TLC or GC/MS (Microgenics, 2016).” Many false-positives can occur due to the high number of cross-reactants, including over-the-counter medicines and dietary supplements (Hoffman, 2022; Moeller et al., 2017). Even metformin, a medication prescribed to treat diabetes, can give false-positives although the mechanism of cross-reactivity is unknown (Fucci, 2012).
Phencyclidine
Phencyclidine (PCP), a N-methyl-D-aspartic acid (NMDA) receptor antagonist, is a dissociative anesthetic that can be abused for its euphoric properties. Also known as angel dust, PCP was the first non-natural man-made DOA (Bertron et al., 2018). Throughout the 1980s and 1990s, the use of PCP declined considerably; however, the Drug Abuse Warning Network has reported a 400% increase in emergency room visits due to PCP use in 2005 – 2011 (Moeller et al., 2017). PCP is typically screened using an immunoassay, and qualitative screening tests, such as CEDIA®, report a 100% reactivity at a PCP concentration of 25 ng/mL (Microgenics, 2015). Unfortunately, many compounds can interfere with the PCP immunoassay, including tramadol (Ly et al., 2012), dextromethorphan, alprazolam, clonazepam, and carvedilol (Rengarajan & Mullins, 2013). Some have reported that diphenhydramine (Benadryl®) also yields false-positive results (Brahm et al., 2010; Levine & Smith, 1990), but other studies have reported it to be statistically insignificant (Rengarajan & Mullins, 2013). The FDA approved Wondfo Phencyclidine Urine Test is an immunochromatographic assay which can identify PCP in human urine with a cutoff of 25 ng/mL (FDA, 2019). Nonetheless, this is considered a preliminary testing method and results should be confirmed with gas chromatography/mass spectrometry (GC/MS) techniques.
Marijuana/THC/Cannabinoids
According to the CDC, the most recent National Survey on Drug Use and Health (NSDUH), conducted by SAMHSA in 2013, showed that approximately 7.5% of people 12 years and older in the U.S. were current users of marijuana, which was up from 5.8% from 2007 (CDC, 2015). Moreover, the CDC reports that the Monitoring the Future (MTF) survey of 8th, 10th, and 12th graders in the U.S. shows that the rate of marijuana usage has remained steady for more than two decades even though many states and municipalities have changed their legislation. Approximately 5.8% of 12th graders reported daily use of marijuana (CDC, 2018a).
Immunoassays for marijuana do not detect tetrahydrocannabinol (THC) directly because THC rapidly metabolizes in vivo (within hours of use). Instead, these assays detect delta-9-THC, a metabolite, which can remain in either the serum or urine for days to weeks, depending on the extent of exposure (Hoffman, 2022). Older urine immunoassays for marijuana were prone to false-positive results (Altunkaya & Smith, 1990; Rollins et al., 1990), but current testing methods are less prone to false-positives (Hoffman, 2022). Due to the legalization of marijuana in certain locales as well as an increase in the potency of the THC in some strains of marijuana, fear of false-positive results due to second-hand smoke has increased. Recent studies show, though, that this is unlikely. None of the individuals tested positive using an immunoassay with a cutoff level of more than 20 ng/mL provided that the room was well-ventilated. If the room was not ventilated, then 4 of 6 individuals tested positive after 1 hour of exposure if the immunoassay had a cutoff level of 20 ng/mL but only 1 individual tested positive at the federal cutoff level of 50 ng/mL under the same conditions (Cone et al., 2015; Herrmann et al., 2015; Moeller et al., 2017). False positive results for THC have also been caused by medications such as Pantoprazole (Vohra et al., 2019). However, Vohra et al. (2019) completed a small study (n = 12) and found that oral proton pump inhibitors (such as Pantoprazole) did not cause false-positive THC results with the THC One Step Marijuana Test Strip. This test strip is a rapid chromatographic immunoassay which detects the delta-9-THC metabolite at a cutoff concentration of 50 ng/mL (Xlar, 2002).
Cocaine
Cocaine is an alkaloid produced biosynthetically by Erthroxylum coca, which is a plant native to western South America; for thousands of years, South Americans have chewed on the dried coca leaves or consumed coca tea to release cocaine in saliva (Drake & Scott, 2018). Pure cocaine was first isolated in the 1880s and was legal in the United States during the second half of the 19th century (Nelson & Odujebe, 2022). It was once a main ingredient of Coca-Cola. Cocaine is now illegal in the United States; importing coca leaves or coca tea is also illegal in the United States but is legal in other countries. Medicinal use of cocaine is typically limited to use in minor otolaryngologic procedures or as a topical anesthetic (Hoffman, 2022). It has vasoconstrictive properties, making it useful in limiting bleeding during nose and throat surgeries (Nelson & Odujebe, 2022).
Cocaine is a powerful nervous system stimulant and is highly addictive. According to the CDC (2019), cocaine was involved in almost 1 in every 5 overdose-related deaths in the United States in 2017, leading to 14,000 cocaine-related deaths. In 2016, almost 5 million Americans reported regular cocaine use, which was approximately 2% of the population (CDC, 2019).
Cocaine has three main metabolites--benzoylecgonine (>50 %), ecgonine methyl ester (32-49%) and norcocaine (5%) (Nelson & Odujebe, 2022). With benzoylecgonine identified as the major urinary metabolite of cocaine, it is usually tested for in blood, urine, hair, saliva, and meconium. Immunoassays are the most specific technique to detect the cocaine metabolite benzoylecgonine; false-positive results are very uncommon (Hoffman, 2022). Cocaine is metabolized very rapidly and may only be detectable in blood and urine for a few hours; however, benzoylecgonine can be detected in the urine for several days if cocaine use is intermittent or very heavy (Nelson & Odujebe, 2022). Appropriate urine tests distinguish between cocaine use and coca leave/tea use because different metabolites are formed from each. The DRI Cocaine Metabolite Assay, developed by Thermo Fisher, is an FDA-approved enzyme immunoassay that uses a specific antibody to detect benzoylecgonine in urine (FDA, 2018). This immunoassay has a concentration cutoff of 150 ng/mL-300 ng/mL.
Clinical Utility and Validity
For acute clinical management of most patients, DOA monitoring is of limited value. Studies have indicated that in specific settings DOA screening does have value, particularly for drug treatment programs, pain management, and/or psychiatric treatment. A large retrospective study (n = 470 patients) by Michna et al. (2007) showed that 20% of individuals in pain management programs tested positive for illicit substances when random screenings were performed. Further, Knezevic et al. (2017) performed a study showing the effect of urine drug testing on patient compliance. Five hundred patients provided supervised urine toxicology samples, 386 of which were compliant with prescribed medications. The patients were educated about their results, and 77 of the noncompliant patients were tested again. Of these 77 patients, 49 had improved compliance (Knezevic et al., 2017). This supports the previous findings of a smaller study by Jamison and colleagues that reported a significant increase in compliance for high-risk chronic pain patients on opioid therapy when monitored by UDT (Jamison et al., 2010). Another study also supports UDT for patients on long-term opioid therapy by showing that “monitoring both urine toxicology and aberrant behavior in chronic-pain patients treated with opioids identified more problem patients than by monitoring either alone (Katz et al., 2003).”
These findings are considerably more favorable than those of the systematic review conducted by Starrels et al. (2010) of eleven different studies that found substantial variation in reduction of opioid misuse in patients with chronic pain. These researchers discovered that “the proportion of patients with opioid misuse after treatment agreements, urine drug testing, or both varied widely (3% to 43%)” and concluded that “relatively weak evidence supports the effectiveness of opioid treatment agreements and urine drug testing in reducing opioid misuse by patients with chronic pain (Starrels et al., 2010).” Even with the controversy, Christo et al. (2011) recommends using an algorithmic approach for urine drug testing where UDT is used to establish “a baseline measure of risk, as well as monitoring for compliance” (Christo et al., 2011), an approach also supported by the Texas Pain Society (Owen et al., 2012).
Additionally, other scenarios may utilize DOA testing to alter medical management. Patients with seizure disorders, such as epilepsy, who are on antiepileptic medications that block sodium channels (including phenytoin, lamotrigine, and carbamazepine) could benefit from DOA testing since cocaine can interact pharmacokinetically with these drugs (Smith & McBride, 1999; Wilfong, 2021). DOA screening to check for cocaine can be used prior to administration of beta-adrenergic antagonists. For patients who exhibit acute psychosis with no apparent or known cause, DOA screening can be used to detect possible stimulants (Hoffman, 2022; McClellan & Stock, 2013). Alternatively, psychiatric pre-administration acetaminophen or salicylate screening is deemed unnecessary by Farkas et al. (2021) following their multicenter retrospective study. The authors analyzed 33,439 tests over 10 years from three different Veteran’s Administration emergency departments. There were no toxicity diagnoses. The authors suggest that the testing is “unnecessary and wasteful” (Farkas et al., 2021).
For monitoring a drug therapy regimen, some have proposed using quantitative, definitive testing (Couto et al., 2009, 2011; Kell, 1994; Pesce et al., 2012). Small studies by Couto and colleagues reported concordance correlation coefficients of 0.677 (n = 20) for assessing adherence to a hydrocodone regimen and 0.689 (n = 36) for an oxycontin regimen using normalized algorithms (Couto et al., 2009, 2011). Other studies have shown that due to the variability in pharmacokinetics, pharmacodynamics, and pharmacogenetics between individuals, such quantitative testing does not correlate to “patient compliance with a drug dosage using commercial algorithms” (Nafziger & Bertino, 2009). Another study by McEvoy et al. (2014) aiming to assess urine levels of aripiprazole and its metabolites for patients on an aripiprazole regimen, at best, only found an R2 value of 0.7 even when adjusted for age, weight, sex, urine creatinine values, height, urine specific gravity, and dosage range. “Unadjusted urine levels of aripiprazole and metabolites are not strongly related to aripiprazole dosing…variance in urine metabolite levels accounted for by medication dose was relatively low for each individual drug/metabolite, [R2] only 0.13 to 0.23 (McEvoy et al., 2014).” Even the study by Couto notes the limitations concerning pharmacogenetics, excluding any patient who was “determined to be poor, rapid, or ultra-rapid CYP2D6 metabolizers” (Couto et al., 2011).
A study performed by Snyder et al. (2017) assessed the accuracy of enzyme immunoassays (EIAs) for patients being treated for chronic pain. A total of 530 patient samples were taken, and the immunoassays were evaluated for accuracy. The EIAs showed an overall sensitivity of 78.5% (detecting 543 of the 692 LC-MS/MS positives). Unfortunately, “21% of EIA for opiates show false negative results.” The authors conclude, “LC-MS/MS methods are superior in terms of sensitivity and number of compounds that can be screened, making this a better method for use in pain management” (Snyder et al., 2017).
A retrospective chart review was conducted by Vopat et al. (2020) for a community-based practice, where 166 patients were examined. Motivated by studies that showed increases in post-operative orthopedic complications associated with pre-operative opioid use, the authors set out to determine whether urine drug screening (UDS) could be an effective screening tool for detecting opioid and illicit drugs prior to joint arthroplasty procedures. In the review, positive UDS results were compared to self-reported history of prescribed opioids. The authors demonstrated using four drug panels that of the 166 patients screened with UDS, 64 (38.6%) tested positive for opiate/opioids, while 7 (4.2%) tested positive for amphetamines, 6 (3.6%) for cannabinoids, and 2 (1.2%) for other drugs, with one participant testing positive across multiple panels. However, it was also admitted that the study may have limited power, given that the population came from a single clinic with a limited number of cases. The narrow detection time of using urine detection screening also presents an issue; for example, drugs such as oxycodone may not be detected if administered more than three days before testing, leading to underestimation. Moreover, the data was not normalized for duration and dosage of opioid use, which are believed to contribute to clinical outcomes. However, the authors ultimately concluded that “With a significant number of patients testing positive for opioids without evidence of a previous prescription, UDS may be beneficial for initial risk assessment for patients undergoing JA procedures” (Vopat et al., 2020).
Palamar et al. (2019) completed research to determine the effectiveness of hair versus urine testing to detect or validate drug use. Data from 532 adults was used in this study. All participants reported using heroin or a nonmedical prescription opioid in the past month. Urine samples were obtained from all participants and almost 80% of participants provided hair samples. “Compared to hair testing, urine testing was able to confirm higher proportions of self-reported use of heroin/opioids (85.5% vs. 80.9%), marijuana (73.9% vs. 22.9%), benzodiazepines (51.3% vs. 15.1%), and methadone (77.0% vs. 48.7%), while hair testing was more likely to detect reported cocaine use (66.3% vs. 48.0%) (Ps<.01). Compared to hair testing, urine testing was more likely to detect unreported use of marijuana (11.3% vs. 0.9%), and benzodiazepines (14.4% vs. 5.4%), and hair testing was more likely to detect unreported use of cocaine (27.0% vs. 5.8%) and oxycodone (19.7% vs. 1.4%)” (Palamar et al., 2019). When used together, hair testing increased the detection of cocaine and/or oxycodone use from 14% to 22%. This is not surprising as cocaine is metabolized very quickly and may be undetectable in urine within hours to a few days depending on use (Nelson & Odujebe, 2022).
Böttcher et al. (2019) evaluated the analytical findings in oral fluid after oral fluid heroin intake. The study used 6-acetylmorphine (6-AM) as the target analyte. 2814 samples from 1875 patients were included. At a cut-off of 1 ng/mL “neat” (undiluted) oral fluid, 406 samples contained at least one opiate in the drug screening. 314 of these samples had a measured 6-AM concentration of ≥ 1 ng/mL. The authors also noted that the positive rates for opiates in oral fluid and urine were identical at 13.5% (in similar populations of patients). The authors concluded that 6-AM “… makes OF drug testing for detecting heroin use more effective than urine drug testing when using highly sensitive mass spectrometry methods” (Böttcher et al., 2019).
A study by Krasowski et al. (2020) used data from a College of American Pathologists survey on urine drug testing and screening proficiency to greater understand the strengths and weaknesses of immunoassays in drug testing. The authors note that there is a strong clinical interest for urine drug testing, and that both opiate and amphetamine immunoassays were highly variable regarding cross-reactivity for drugs other than the actual assay calibrator. The authors also found that “urine drug testing availability does not parallel prevailing patterns of drug prescribing and abuse patterns. In particular, specific immunoassays for synthetic opioids and a lower positive cutoff for opiate immunoassays may be underused, whereas immunoassays for barbiturates, methadone, propoxyphene, and phencyclidine may be overused” (Krasowski et al., 2020).
Argoff et al. (2018) published consensus report regarding “urine drug monitoring (UDM) in patients with chronic pain who are prescribed opioids.” It is important to note that this publication was sponsored by major toxicology laboratories. The specialists convened were “an interdisciplinary group of clinicians with expertise in pain, substance use disorders, and primary care”. They have issued recommendations based on their review of relevant literature, existing guidelines, and their clinical experiences in UDM. Their relevant recommendations are listed below:
- “Use definitive UDM testing (e.g., with GC-MS, LC-MS, or LC-MS/MS) as the most accurate method for assessing baseline opioid use and opioid misuse in almost all patients with chronic pain being considered for opioids as well as for ongoing monitoring of patients receiving opioids for chronic pain, unless presumptive testing is required by institutional or payer policies.” The guideline acknowledges that “The recommendations in this consensus are intended to be considered together with practical clinical and payer concerns. When required by payers and institutions, immunoassays may be sufficient for monitoring low-risk patients, particularly when clinicians and patients engage in open communication.”
- “Perform UDM at baseline in patients prescribed opioids for chronic pain. During ongoing monitoring, perform UDM at least annually for low-risk patients, two or more times per year for moderate-risk patients, and three or more times per year for high-risk patients. Additional monitoring can be performed at any risk level as frequently as necessary according to clinical judgment (Argoff et al., 2018)”.
Several organizations recognize the benefit of drug screening/testing for the identification and management of drug misuse and abuse; however, standard guidelines for who should be tested, what test should be used, and how frequently testing should occur, are lacking.
Centers for Disease Control and Prevention (CDC)
In 2016, the CDC published guidelines for prescribing opioids for chronic pain (Dowell et al., 2016). Within the guidelines, the CDC recommends that clinicians should consider urine drug testing prior to discontinuing opioids to determine possibility of withdrawal. The CDC also recommends that “when prescribing opioids for chronic pain, clinicians should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications as well as other controlled prescription drugs and illicit drugs (recommendation category: B, evidence type: 4).” The CDC states that “urine drug tests can provide information about drug use that is not reported by the patient. In addition, urine drug tests can assist clinicians in identifying when patients are not taking opioids prescribed for them, which might in some cases indicate diversion or other clinically important issues such as difficulties with adverse effects” (Dowell et al., 2016).
Concerning the frequency of urine drug testing, they state, “While experts agreed that clinicians should use urine drug testing before initiating opioid therapy for chronic pain, they disagreed on how frequently urine drug testing should be conducted during long-term opioid therapy. Most experts agreed that urine drug testing at least annually for all patients was reasonable. Some experts noted that this interval might be too long in some cases and too short in others, and that the follow-up interval should be left to the discretion of the clinician. Previous guidelines have recommended more frequent urine drug testing in patients thought to be at higher risk for substance use disorder. However, experts thought that predicting risk prior to urine drug testing is challenging and that currently available tools do not allow clinicians to reliably identify patients who are at low risk for substance use disorder” (Dowell et al., 2016).
The CDC also published a guideline “Quality Improvement and Care Coordination: Implementing the CDC Guideline for Prescribing Opioids for Chronic Pain” to provide guidance to healthcare systems and practice leaders. In it, the CDC details specific procedures to take when “unexpected results” appear:
- When the UDT is negative for a prescribed opioid, the CDC recommends repeating the test “using chromatography” and to specify the drug of interest.
- When the UDT is positive for a non-prescribed opioid, benzodiazepines, or illegal drugs, the CDC recommends repeating the UDT regularly.
- When the urine sample has a creatinine level of < 2 – 3 mmol/L or < 20 mg/dL, the CDC recommends repeating the UDT.
- When the urine sample is cold, the CDC recommends repeating the UDT (CDC, 2018b).
American Academy of Family Physicians (AAFP)
The AAFP published in 2019 recommendations concerning ordering and interpreting urine drug tests. They state, “Several federal and state regulations have been enacted that recommend or require urine drug testing in patients receiving long-term opioid therapy. Similar guidance may apply to patients receiving long-term benzodiazepine or stimulant therapy” (Kale, 2019). They state that the frequency of urine drug testing depends on individual risk factors and is ultimately left to the attending physician; however, they do state a recommended frequency for urine drug testing given in the table below:
|
Recommended Frequency for Urine Drug Testing (Kale, 2019) |
|
|
Level of misuse risk |
Frequency of testing |
|
Low (no risk factors) |
Every 6 to 12 months |
|
Moderate |
Every 3 to 6 months |
|
High (mental health disorder, substance use disorder, prior opioid misuse, aberrant behavior*) or opioid dosage > 120 morphine milligram equivalents |
Every 1 to 3 months |
|
*Aberrant behavior includes, but is not limited to, lost prescriptions, multiple requests for early refills, opioid prescriptions from multiple physicians, unauthorized dose escalation, and apparent intoxication. |
|
They state the following clinical recommendation: “Urine drug testing can be used to monitor compliance with prescribed therapy and detect the use of nonprescribed and illicit substances, especially opioids, benzodiazepines, and heroin”.
In 2020, the AAFP provided a clinical preventive service recommendation on screening for opioid use disorder, stating that “The AAFP recommends that clinicians selectively screen and refer adults aged 18 years and older to OUD treatment after weighing the benefits and harms of screening and treatment. Clinicians should consider all benefits and harms including health, social, and legal outcomes. Screening programs should only be implemented if services for accurate diagnosis, effective treatment, and psychosocial supports can be offered or referred.” This recommendation falls under the category of grade C, or the recommendation provides “at least moderate certainty that the net benefit is small” (AAFP, 2020).
Federation of State Medical Boards (FSMB)
The FSMB indicates in their Guidelines for Chronic Use of Opioid Analgesics policy that for patients being prescribed opioids for chronic pain management that the initial workup should include a system review and relevant physical examination, as well as laboratory investigations as indicated (FSMB, 2017). They also note the utility of periodic and unannounced testing for monitoring adherence to the patient’s treatment plan and to detect non-prescribed drugs. Regarding frequency of testing, “Patients being treated for addiction should be tested as frequently as necessary to ensure therapeutic adherence, but for patients being treated for pain, clinical judgment trumps recommendations for frequency of testing” (FSMB, 2017).
Additionally, relative to how testing should be performed, the Federation of State Medical Boards notes that POC tests have significant limitations in both sensitivity and specificity, and therefore “the use of point of care testing for the making of more long term and permanent changes in management of people with the disease of addiction and other clinical situations may not be justified until the results of confirmatory testing with more accurate methods … are obtained.” They do state, “Urine may be the preferred biologic specimen for testing because of its ease of collection and storage and the cost-effectiveness of such testing. When such testing is conducted as part of pain treatment, forensic standards are generally not necessary and not in place” (FSMB, 2017). They also note that initial testing could be done using immunoassays and followed up by a more specific technique, such as GC/MS or other chromatography-based technique. They highlight the importance of knowing specific drug and metabolites, “not just the class of drug” for the pain management.
American Academy of Pain Medicine
The AAPM notes that “urine and/or blood drug screening … may be helpful in ruling out the issue of diversion,” along with other non-testing actions. They also note that “when appropriate, the patient should undergo a baseline drug screening exam.” They highlight the importance of random urine drug screening for the ongoing monitoring of patient compliance to the treatment plan (AAPM, 2013).
The AAPM also co-sponsored guidelines with the American Association for Clinical Chemistry in 2018. These guidelines by Langman and Jannetto (2018) are shown below.
American Association for Clinical Chemistry (AACC)
In 2017, the AACC published their guidelines titled Using Clinical Laboratory Tests to Monitor Drug Therapy in Pain Management Patients (Jannetto et al., 2017). These guidelines were reaffirmed in 2018 and co-sponsored by the AAPM (Langman & Jannetto, 2018). The AACC lists medications in tiers to guide ordering of tests. Tier 1 is “routine monitoring” and includes frequently abused drugs as well as drugs frequently prescribed to pain management patients. Benzodiazepines, amphetamines, and barbiturates are in this tier. Anticonvulsants and antidepressants fall in tier 2, which is as follows: “High-risk patients with known history of abuse for this medication or prevalence of drug use is endemic to local region, risky polypharmacy, multiple providers, or if prescribed and patient shows lack of efficacy or toxicity” (Jannetto et al., 2017). Antipsychotics fall in tier 3: which should be ordered “as clinically indicated.”
The NACB [AACC] lists their recommendations with a grade for the quality of evidence as well as the strength of recommendation. An A represents a strong recommendation, a B is moderate recommendation, and C is a recommendation against. For the quality of evidence, an “I” represents “consistent results from well-designed, well-conducted studies in representative populations” whereas an “II” means “Evidence is sufficient to determine effects, but the strength of the evidence is limited by the number, quality, or consistency of the individual studies; generalizability to routine practice; or indirect nature of the evidence.” The NACB’s recommendations are as follows (Jannetto et al., 2017; Langman & Jannetto, 2018):
- “Testing biological specimens for drugs/drug metabolites is recommended and effective for detecting the use of relevant over-the-counter, prescribed and non-prescribed drugs, and illicit substances in pain management patients. Laboratory testing does not specifically identify most other outcomes, but should be used in conjunction with additional information to detect other outcomes in pain management patients. Strength of Recommendation: A; Quality of Evidence: I”
- “More frequent laboratory testing is recommended for patients with a personal or family history of substance abuse, mental illness, evidence of aberrant behavior, or other high-risk characteristics. Strength of Recommendation: A; Quality of Evidence: II”
- “Laboratory testing is recommended to identify the use of relevant over-the-counter medications, prescribed and non-prescribed drugs, and illicit substances in pain management patients. However, it does not effectively identify all non-compliance with the prescribed regimen. No single monitoring approach provides adequate information about the pattern or dose of patient drug use. Safest prescribing habits should include a combination of tools and laboratory test results to correctly detect outcomes. Strength of recommendation: A; Quality of evidence: III (pain management population), II (substance abuse disorder monitoring population)”
- “Laboratory testing is more effective than other physician tools for the detection of relevant over-the-counter, prescribed and non-prescribed drugs, and illicit substances in pain management patients and should be used routinely to monitor compliance. Strength of recommendation: A; Quality of evidence: II”
- “Urine testing is recommended for the detection of relevant over-the-counter medications, prescribed and nonprescribed drugs, and illicit substances in pain management patients. Strength of recommendation: B; Quality of evidence: II”
- “Based on level II evidence, baseline drug testing should be performed prior to initiation of acute or chronic controlled substance therapy. In addition, random drug testing should be performed at a minimum of one to two times a year for low-risk patients (based on history of past substance abuse/addiction, aberrant behaviors, and opioid risk screening criteria), with increasing frequency for higher-risk patients prescribed controlled substances. Strength of Recommendation: A; Quality of Evidence: II”
- “Serum or plasma is an acceptable alternate matrix for the detection of relevant over-the-counter medications, prescribed and non-prescribed drugs, and illicit substances in pain management patients with end-stage renal failure (anuria). For dialysis patients, the blood (serum/plasma) should be collected prior to dialysis. Oral fluid testing can also be used for selected drugs (e.g. amphetamine, benzodiazepines, buprenorphine, tetrahydrocannabinol, cocaine, codeine, hydrocodone, hydromorphone, methadone, morphine, oxycodone, and oxymorphone). Strength of recommendation: A; Quality of evidence: III”
- “While definitive testing is recommended and preferred, urine immunoassays performed on laboratory-based analyzers offer some clinical utility to detect the use of relevant over-the-counter medications, prescribed and non-prescribed drugs, and illicit substances in pain management patients. However, physicians using immunoassay-based tests (especially amphetamine, benzodiazepine, and opiate immunoassays) must reference the package insert if testing in the physician’s office or consult with laboratory personnel to evaluate the assay’s capabilities and limitations for detecting specific medications within a drug class to prevent incorrect interpretation and to determine when additional testing is necessary. Strength of Recommendation: B; Quality of Evidence: II”
- “Qualitative definitive tests should be used over immunoassays since they are more effective at identifying relevant over-the-counter medications, prescribed and non-prescribed drugs, and illicit substances in pain management patients. Strength of Recommendation: A; Quality of Evidence: II”
- “Qualitative definitive tests should be used when possible over immunoassays for monitoring use (compliance) to relevant over-the-counter medications, prescribed and non-prescribed drugs, and illicit substances in pain management patients due to their superior sensitivity and specificity. Strength of Recommendation: A; Quality of Evidence: II”
- “POC (oral/urine) qualitative presumptive immunoassays offer similar performance characteristics to laboratory-based immunoassays and can detect some over-the-counter medications, prescribed and non-prescribed drugs, and illicit substances in pain management patients. However, physicians using POC testing must reference the POC package insert and/or consult laboratory personnel to accurately determine the assay’s capabilities (especially amphetamine, benzodiazepine, and opiate immunoassays) and understand the limitations for detecting specific medications within a drug class to prevent incorrect assumptions or interpretation and to determine when additional testing is necessary. Strength of Recommendation: B; Quality of Evidence: II”
- “Qualitative immunoassay drug testing prior to prescribing controlled substances can be used to identify some illicit drug use and decrease adverse outcomes in pain management patients. Strength of Recommendation: B; Quality of Evidence: II”
- “Random urine testing for relevant over-the-counter medications, prescribed and non-prescribed drugs, and illicit substances is recommended to detect outcomes in pain management patients. Strength of Recommendation: A; Quality of Evidence: III (pain management population), II (substance abuse disorder monitoring population)”
- “Appropriately performed and interpreted urine POC immunoassay testing can be cost-effective for detecting use or inappropriate use of some over-the-counter medications, prescribed and non-prescribed drugs, and illicit substances in pain management patients. Strength of Recommendation: B; Quality of Evidence: II”
- “Firstline definitive testing (qualitative or quantitative) is recommended for detecting the use of relevant over-the-counter medications, prescribed and non-prescribed drugs, and illicit substances in pain management patients. Strength of recommendation: A; Quality of evidence: II”
- “Recommend definitive testing for any immunoassay (laboratory-based or POC) result that isn’t consistent with the clinical expectations in a pain management patient. Strength of recommendation: A; Quality of evidence: III”
- “Quantitative definitive urine testing is not more useful at detecting outcomes in pain management patients compared to qualitative definitive urine testing. Furthermore, quantitative definitive urine testing should not be used to evaluate dosage of administered drug or adherence to prescribed dosage regimen. However, quantitative urine definitive testing is recommended to identify variant drug metabolism, detect pharmaceutical impurities, or metabolism through minor routes. Quantitative results may also be useful in complex cases to determine the use of multiple opioids, confirm spiked samples, and/or rule out other sources of exposure (e.g. morphine from poppy seeds). Strength of recommendations: A; Quality of evidence: II”
- “The use of lower limit-of-detection cutoff concentrations can be more effective to detect use (either partial or full compliance) or the lack of use of relevant over-the-counter medications, prescribed and non-prescribed drugs, and illicit substances in pain management patients, especially those taking lower dosages. Strength of Recommendation: B; Quality of Evidence: II” (Jannetto et al., 2017; Langman & Jannetto, 2018).
American Pain Society/American Academy of Pain Medicine
The American Pain Society and American Academy of Pain Medicine joint guidelines panel released their opioid treatment guidelines titled Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Non-cancer Pain in 2009. They addressed the monitoring of controlled substances use via UDT as part of a chronic opioid treatment (COT) program. The authors recommend periodic urine drug screening and suggest that random urine drug screens may be more informative than scheduled or routine testing. The guideline section on monitoring (Section 5) states:
- “5.1: Clinicians should reassess patients on COT periodically and as warranted by changing circumstances. Monitoring should include documentation of pain intensity and level of functioning, assessments of progress toward achieving therapeutic goals, presence of adverse events, and adherence to prescribed therapies (strong recommendation, low-quality evidence).
- 5.2: In patients on COT who are at high risk or who have engaged in aberrant drug-related behaviors, clinicians should periodically obtain urine drug screens or other information to confirm adherence to the COT plan of care (strong recommendation, low-quality evidence).
- 5.3: In patients on COT not at high risk and not known to have engaged in aberrant drug-related behaviors, clinicians should consider periodically obtaining urine drug screens or other information to confirm adherence to the COT plan of care (weak recommendation, low-quality evidence). Clinicians should periodically reassess all patients on COT. Regular monitoring of patients once COT is initiated is critical because therapeutic risks and benefits do not remain static” (Chou et al., 2009).
The American Pain Society guidelines state that for individuals at low risk for adverse outcomes, quarterly or semi-annual monitoring is sufficient. The risk for abuse may be measured using standard tools, such as the Screener and Opioid Assessment for Patients with Pain (SOAPP) and the Opioid Risk Tool. These types of tools may help clinicians assess the suitability of long-term opioid therapy for chronic pain patients and may help differentiate those patients who require more clinician monitoring while on long-term opioid therapy. Both tools may be self-administered at or prior to an office visit, or completed as part of an interview with a nurse, physician or psychologist (Chou et al., 2009).
American Society of Interventional Pain Physicians (ASIPP)
ASIPP issued evidence-based clinical practice guidelines to improve the quality of care through responsible opioid prescribing in non-cancer pain. They have described evidence assessment followed in Part 1 of the guidelines and the recommended guidance in Part 2.
ASIPP provides 11 recommendations including drug cut-offs and detection limits for drugs of abuse, drug cross-reactants, guidance on interpretation of unexpected results for urine drug testing and urine drug testing algorithm. In their algorithm, ASIPP proposes to perform baseline assessment of the patient with chronic pain using POC immunoassay. Then, depending on the result to continue either compliance monitoring with random POC immunoassay in 1 – 3 months if initial results were appropriate or explained, followed-up with random testing in 6 – 12 months if the result remains appropriate. In the case when inappropriate or unexplained results are obtained, confirmatory testing is proposed with repeat urine drug testing in one month or next appointment (Manchikanti et al., 2012).
In their recommendation 1D, level of evidence good, ASIPP states: “Urine drug testing (UDT) must be implemented from initiation along with subsequent adherence monitoring to decrease prescription drug abuse or illicit drug use when patients are in chronic pain management therapy.” Additionally, they state, “In order to reduce prescription drug abuse and doctor shopping, adherence monitoring by UDT and PMDPs provide evidence that is essential to the identification of those patients who are non-compliant or abusing prescription drugs or illicit drugs.” Level of evidence is fair (Manchikanti et al., 2012).
A 2017 update from ASIPP reaffirms the use of urine drug testing and monitoring programs when taking the initial steps towards opioid therapy, captured below.
“1. Comprehensive assessment and documentation. (Evidence: Level I; Strength of Recommendation: Strong)
2. Screening for opioid abuse to identify opioid abusers. (Evidence: Level II-III; Strength of Recommendation: Moderate)
3. Utilization of prescription drug monitoring programs (PDMPs). (Evidence: Level I-II; Strength of Recommendation: Moderate to strong)
4. Utilization of urine drug testing (UDT). (Evidence: Level II; Strength of Recommendation: Moderate)
5. Establish appropriate physical diagnosis and psychological diagnosis if available. (Evidence: Level I; Strength of Recommendation: Strong)
6. Consider appropriate imaging, physical diagnosis, and psychological status to collaborate with subjective complaints. (Evidence: Level III; Strength of Recommendation: Moderate)
7. Establish medical necessity based on average moderate to severe (? 4 on a scale of 0 – 10) pain and/or disability. (Evidence: Level II; Strength of Recommendation: Moderate)
8. Stratify patients based on risk. (Evidence: Level I-II; Strength of Recommendation: Moderate)
9. Establish treatment goals of opioid therapy with regard to pain relief and improvement in function. (Evidence: Level I – II; Strength of Recommendation: Moderate)
10. Obtain a robust opioid agreement, which is followed by all parties. (Evidence: Level III; Strength of Recommendation: Moderate) (Manchikanti et al., 2017)”.
Monitoring may also continue for adherence and side effects, extending through the final phases:
“19. Monitor for adherence, abuse, and noncompliance by UDT and PDMPs. (Evidence: Level I – II; Strength of Recommendation: Moderate to strong)
20. Monitor patients on methadone with an electrocardiogram periodically. (Evidence: Level I; Strength of Recommendation: Strong).
21. Monitor for side effects including constipation and manage them appropriately, including discontinuation of opioids when indicated. (Evidence: Level I; Strength of Recommendation: Strong)
iv. Final Phase
22. May continue with monitoring with continued medical necessity, with appropriate outcomes. (Evidence: Level I-II; Strength of Recommendation: Moderate)
23. Discontinue opioid therapy for lack of response, adverse consequences, and abuse with rehabilitation. (Evidence: Level III; Strength of Recommendation: Moderate) (Manchikanti et al., 2017)”.
Agency Medical Directors' Group (AMDG)
The Washington State AMDG published an Interagency Guideline on opioid dosing for chronic non-cancer pain. This guideline and related expert commentary support low-risk individuals having UDT up to once per year, moderate risk up to two per year, high risk individuals up to 3 – 4 tests per year, and individuals exhibiting aberrant behaviors should be tested at the time of the office visit (AMDG, 2015).
Supplemental guidance on prescribing opioids for postoperative pain was published by the AMDG in 2018. Specific opioid testing methods are not mentioned in these guidelines (AMDG, 2018).
Wisconsin Worker’s Compensation Patient Care
Wisconsin’s Worker’s Compensation program recommends for any worker’s compensation patient who will need opioid treatment for a period of more than 90 days, that the treating physician should follow these guidelines and or consider referral to a Pain Management specialist. In their document, they state that “urine drug screening before starting chronic opioid therapy is imperative” to verify that patient is not using illegal substances. In addition, according to their guidelines, compliance monitoring is mandatory for all patients on chronic opioid therapy with several tools including urine drug screen for the first visit and with aberrant behavior and unannounced urine drug screens thereafter (DWD, 2013).
American Society of Addiction Medicine (ASAM)
ASAM states quantification (assessing specific concentration of a drug) should not be used to determine adherence with a specific dosage or formulation regimen. There are, however, specific reasons for obtaining quantitative data. For example, quantification can help a clinician decide why the other opioids are present. Serial creatinine-corrected quantitative values can help the clinician distinguish cessation of drug use from continued drug excretion from ongoing drug use. Finally, the guidelines note that state laws may also guide testing decisions (ASAM, 2013).
In 2017, the ASAM recommended drug testing as “an important supplement to self-report because patients may be unaware of the composition of the substance(s) they have used” (Jarvis et al., 2017). They also recommend to not rely on the SAMHSA-5 panel as a routine drug panel. ASAM states that urine testing for amphetamines and benzodiazepines may be helpful when assessing potential use. The society also emphasizes that the results must be carefully analyzed due to specificity limitations in both immunoassays.
With regards to general testing, ASAM recommends random, unannounced testing as opposed to scheduled ones. They recommend, “presumptive testing should be a routine part of initial and ongoing patient assessment.” Concerning definitive drug testing, they recommend, “Definitive testing techniques should be used whenever a provider wants to detect specific substances not identified by presumptive methods, quantify levels of the substance present, and refine the accuracy of the results. Definitive testing should be used when the results inform clinical decisions with major clinical or nonclinical implications for the patient (e.g. treatment transition, changes in medication therapies, changes in legal status)” (Jarvis et al., 2017). ASAM also considers GC/MS and LC-MS testing for confirmation of a presumptive positive test. For patients in substance abuse treatment, ASAM recommends frequent random testing (at least weekly) initially. Once the patient is stable in treatment, then the frequency can decrease (to at least monthly).
New York State Office of Addiction Services and Supports (OASAS)
The OASAS published guidelines on toxicology testing during treatment for substance use disorders. The guidelines specify that toxicology testing may include urine, blood, breath, oral fluid, sweat, and hair, but note that urine testing is the come common and validated method.
The guidelines outline when toxicology testing should be completed. Toxicology testing should be used during initial diagnosis to determine which substances have been used recently and to guide further clinical decisions and testing. Toxicology testing should also be used to determine the level of care necessary, monitor treatment progress, monitor the use of secondary substances, to determine if symptoms are related to a substance, and when documentation of current substances is needed. Lastly, toxicology testing should be used for “return to use prevention” to help the patient make informed decisions.
The guidelines go on to outline how often toxicology tests should be administered. Toxicology testing should be completed upon patient intake, as requested by the patient, at random intervals throughout treatment (decreasing in frequency), and as clinically needed. The guidelines note that the window of detection for each substance must be considered when determining toxicology testing frequency. Participants in the New York State Drug Courts may require toxicology testing twice a week. Patients in opioid treatment programs require “at least eight random toxicology tests per year” (OASAS, 2021).
Texas Pain Society
The Texas Pain Society released detailed guidelines concerning urine drug testing (UDT) and its use in the practice of pain management. They do not recommend a prescribed regimen of UDT but rather leave it to the discretion of the physician. They do recommend random UDT over scheduled UDT. Concerning what should be included in a UDT, “Elements of UDT may include specific gravity, temperature at the time of sample collection, pH, creatinine concentration, and mass spectroscopic confirmatory testing for the following agents: opioids (fentanyl, oxycodone, oxymorphone, tramadol, methadone, hydrocodone, hydromorphone, morphine, codeine, propoxyphene, meperidine, buprenorphine, tapentadol, 6-mono-acetyl morphine [6-MAM]) …”(Owen et al., 2012). Concerning the frequency of conducting UDTs, they recommend 1 – 2 tests per year for low-risk patients; 3 – 4 tests per year for moderate-risk patients; and “4 [per year] or every month, office visit, or every drug refill” for high-risk patients.
2014 Annals of Internal Medicine Review
In 2014 Nuckols and colleagues released an extensive review of guidelines on prescribing and monitoring opioids from more than 10 different societies and organizations in the Annals of Internal Medicine. No consensus concerning urine drug monitoring or testing was noted across all guidelines; in fact, the APS-AAPM noted to use UDT only “if risk is high; consider otherwise.” The NOUGG recommends that, if UDT is used, to consider pros and cons (expert consensus). The Colorado Division of Workers Compensation requires mandatory UDT. The VA/DoD and ASIPP uses UDT to establish a baseline followed by random testing during treatment whereas the ACOEM and UMHS uses UDT to establish a baseline followed by either a minimum of quarterly testing or annual testing, respectively (Nuckols et al., 2014).
Substance Abuse and Mental Health Services Administration (SAMHSA)
These guidelines are for the certification for opioid treatment programs (OTPs). OTPs require certification before they can dispense opioids to treat opioid addiction. SAMHSA recommends benzodiazepines and amphetamines at a minimum be tested before admission to any opioid treatment program. Barbiturates are also strongly recommended to be tested at regular intervals during the program. Testing is not limited to these classes of drugs and may vary from individual to individual (SAMHSA, 2007).
SAMHSA federal guidelines for opioid treatment programs were updated in 2015. These guidelines state that “Clinicians should determine the drug-testing regimen by analyzing community drug-use patterns and individual medical indications. It is strongly recommended that benzodiazepines, barbiturates, and alcohol (using the ethyl glucuronide test) be included in drug screening and testing panels (SAMHSA, 2015, 2017).” The guidelines also state that “OTPs often perform onsite point of collection (POC) tests using sensitive and automated immunoassay (IA) technologies that screen urine or oral fluid samples for a relatively narrow range of drug classes (e.g. amphetamines, barbiturates, benzodiazepines, opioids) and a limited number of specific drugs. POC tests such as IAs have a place in clinical decision making, but are not by themselves adequate to satisfy the regulatory requirements for drug use testing services” (SAMHSA, 2015).
In 2020, SAMHSA published guidelines regarding use of oral fluid for federal workplace drug testing programs. In it, they remarked that “The Department believes that collecting and testing oral fluid specimens according to the requirements in these Guidelines is an efficient means to detect illicit drug use and ensures that the oral fluid test results are forensically and scientifically supportable.” SAMHSA writes that several reasons demanded the need for regulation of oral fluid testing, such as the need to decrease invalid urine tests. SAMHSA writes that an oral fluid specimen may be used for the following reasons: “a federal agency applicant/preemployment test, a random test, a reasonable suspicion/cause test, a post-accident test, a return to duty test, or a follow-up test” (SAMHSA, 2020).
Regarding the tests that should be conducted on an oral fluid specimen, a federal agency:
“(a) Must ensure that each specimen is tested for marijuana and cocaine as provided in the drug testing panel described under Section 3.4;
(b) Is authorized to test each specimen for other Schedule I or II drugs as provided in the drug testing panel;
(c) Is authorized upon a Medical Review Officer's request to test an oral fluid specimen to determine specimen validity using, for example, a test for a specific adulterant;
(d) Is authorized to test each specimen for one or more biomarkers as provided in the biomarker testing panel described under Section 3.4; and
(e) If a specimen exhibits abnormal characteristics (e.g., unusual odor or color, semi-solid characteristics), causes reactions or responses characteristic of an adulterant during initial or confirmatory drug tests (e.g., non-recovery of internal standard, unusual response), or contains an unidentified substance that interferes with the confirmatory analysis, then additional testing may be performed” (SAMHSA, 2022).
Section 3.4 refer to drug and biomarker test analytes and cutoffs for undiluted (neat) oral fluids, and a screenshot is included below.
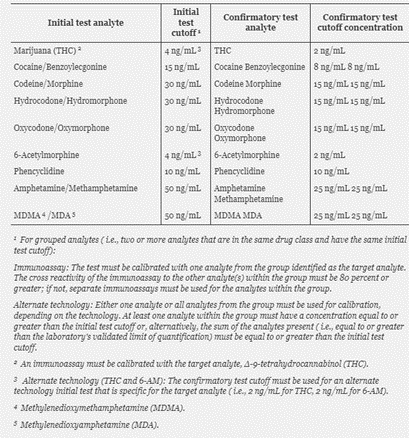
(SAMHSA, 2022)
American Association for the Treatment of Opioid Dependence Inc. (AATOD)
The AATOD recommends cessation of benzodiazepines before admission to an opioid treatment program (OTP). Gradually tapering off to a lower dose is also acceptable, but benzodiazepine use must be addressed prior to an OTP admission. The AATOD recommends toxicology screening for benzodiazepines, as well as routine checks of each state’s Prescription Monitoring Drug Program. Confirmatory testing may also be used (AATOD, 2017).
Department of Health and Human Services (HHS)
The HHS has provided guidelines on Federal Workplace Drug Testing Programs. Federal agencies must comply with these guidelines by October 1, 2017. Each specimen must be tested for marijuana and cocaine metabolites, phencyclidine, opioids, and amphetamines. Validity tests such as creatinine, specific gravity, pH, and oxidizing adulterants must be performed on each specimen. Additional testing must be performed on any abnormal specimens, such as unusual color or smell. Additional drugs may be tested for if the federal agency has reasonable suspicion or if the testing is done post-accident. Additional approval is required. The criteria for an adulterated specimen are as follows: pH < 4 or > 11, nitrite concentration > 500 mcg/mL, chromium concentration >50 mcg/mL, and including but not limited to halogens, surfactants, pyridines. Unusual creatinine or specific gravity may also cause a specimen to be invalid (HHS, 2017).
American Academy of Child and Adolescent Psychiatry (AACAP)
AACAP notes, “Toxicology screens are indicated for acute onset or exacerbations of psychosis when exposure to drugs of abuse cannot otherwise be ruled out. Genetic testing is indicated if there are associated dysmorphic or syndromic features” (McClellan & Stock, 2013).
World Federation of Societies of Biological Psychiatry (WFSBP)
The WFSBP states that drug screening (urine and blood) should be sought for schizophrenia patients as “presence of substance abuse or dependence is often not recognized and systematically assessed, especially if such a patient is seen during an acute psychotic episode” (WFSBP, 2015).
National Institute for Health and Care Excellence (NICE)
NICE notes that appropriate blood tests (such as glucose, calcium, and so on) should be considered in adults to identify potential causes or co-morbidities. NICE also acknowledges that children may be tested to exclude other diagnoses or determine an underlying cause of the epilepsy. They also state, “In children and young people, other investigations, including blood and urine biochemistry, should be undertaken at the discretion of the specialist to exclude other diagnoses, and to determine an underlying cause of the epilepsy” (NICE, 2018, 2020).
American Academy of Neurology (AAN)
The AAN states that “toxicology testing may be considered in children with status epilepticus, when no apparent etiology is immediately identified” (AAN, 2018). These guidelines were reaffirmed in 2022.
Department of Veterans Affairs/Department of Defense (VA/DOD)
In 2015, the VA/DOD issued recommendations surrounding the screening and treatment of substance use disorders. In it, it was recommended that “For patients in general medical and mental healthcare settings, we recommend screening for unhealthy alcohol use annually using the three-item Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) or Single Item Alcohol Screening Questionnaire (SASQ)” (DVA & DOD, 2015).
In 2017, the VA/DOD published clinical practice guidelines for opioid therapy for chronic pain. These guidelines recognize that “UDT and confirmatory testing is an additional method of examining for patient substance misuse” (DOD, 2017). The guidelines also state that “It is critical that the UDT and confirmatory testing be done in a timely, confidential, accurate, and easily available manner to assure the prescribers, patients, and public that safety, fairness, and trust are being addressed (DOD, 2017).” The VA/DOD also recognizes the three main types of UDTs: immunoassay, GC/MS confirmatory testing and LC/MS confirmatory testing.
With respect to antepartum use of alcohol, cigarettes, illicit drugs, and the like, these joint guidelines state “We recommend screening for use of tobacco, alcohol, illicit drugs, and unauthorized use of prescription medication because their use is common and can result in adverse outcomes” (strong recommendation) (DOD, 2018).
Anxiety Disorders Association of Canada (ADAC)
The ADAC recommends urine toxicology as part of the patient’s baseline investigations if warranted. This urine toxicology assessment applies to anxiety and other related disorders, which include “panic disorder, agoraphobia, GAD, selective mutism, separation anxiety disorder, SAD (social phobia), specific phobia, substance/medication-induced anxiety disorder, as well as anxiety disorder due to another medical condition or not elsewhere classified” (Katzman et al., 2014).
American Psychiatric Association, Practice Guidelines for the Psychiatric Evaluation of Adults, 3rd Edition (2016)
The Association acknowledges that urine toxicology may provide clues to substance abuse during an initial psychiatric evaluation (APA, 2016).
World Health Organization (WHO)
The WHO released an intervention guideline for mental, neurological, and substance use disorder in non-specialized health settings. The WHO states that urine testing may be considered to confirm abstinence and to “consider occasional urine testing to confirm non-use.” Under the section concerning the investigation of chronic drug use, they state to consider using urine drug screens “for emergency cases, a urine drug screen should be conducted whenever intoxication, withdrawal, or overdose is suspected, especially in cases when the person is unable to convey what they have ingested” (WHO, 2016). The WHO lists the following substances as psychoactive substances: alcohol, benzodiazepines, opioids, tobacco, cocaine, methamphetamines, amphetamine-type stimulants, khat, cannabis, tramadol, “volatile” solvents, MDMA, and hallucinogens.
American College of Obstetricians and Gynecologists (ACOG)
ACOG states that additional research is needed to better understand the effects of universal urine screening on clinical outcomes and recommend validated verbal screening tools instead. ACOG acknowledges that urine drug testing has been used to identify substance abuse and should only be performed in compliance with state’s laws and with patient consent. ACOG also lists the following recommendations:
- “Screening for substance use should be part of comprehensive obstetric care and should be done at the first prenatal visit in partnership with pregnant woman. Screening based only on factors, such as poor adherence to prenatal care or prior adverse pregnancy outcome, can lead to missed cases and may add to stereotyping and stigma. Therefore, it is essential that screening be universal.”
- “Routine screening should rely on validated screening tools, such as questionnaires, including 4Ps, NIDA, Quick Screen, and CRAFFT (for women 26 years or younger) (ACOG, 2017).
ACOG explicitly states, “Routine urine drug screening is controversial for several reasons. A positive drug test result is not in itself diagnostic of opioid use disorder or its severity. Urine drug testing only assesses for current or recent substance use; therefore, a negative test does not rule out sporadic substance use. … Health care providers should be aware of t heir laboratory’s test characteristics and request that confirmatory testing with mass spectrometry and liquid or gas chromatography be performed as appropriate” (ACOG, 2017).
Society of Obstetricians and Gynaecologists of Canada (SOGC)
The SOGC recommends periodic drug screening for all pregnant women and all women of childbearing age (III-A). The recommended method of drug screening is a urine toxicology screen (II-2A); however, they state that prior to maternal drug toxicology testing is ordered that informed consent be obtained (III-B) (Wong et al., 2011).
Updated 2017 SOGC guidelines state that “When testing for substance use is clinically indicated, urine drug screening is the preferred method (II-2A)” (Ordean et al., 2017).
Canadian Paediatric Society (CPS)
In 2017, the CPS — in a position statement dealing with cannabis in Canada’s children and youth — urged the following recommendation for healthcare providers: “Screen all children and youth for cannabis exposure and/or use and educate adolescents and families on the health risks and harms associated with cannabis” (Grant & Bélanger, 2017).
The CPS within their 2018 guidelines on ADHD in children and youth state, “Children with ADHD may also experience comorbid depressive symptoms, particularly as they approach adolescence and adulthood. There is increasing evidence of heterotypic continuity between these two conditions, suggesting they may represent the same underlying construct for some children. The validity of BD diagnosis, particularly when broadly defined, remains controversial in preadolescent children. … There is an increase in SUDs as children with ADHD reach adolescence and adulthood. It is possible that substance use occurs as an attempt to self-medicate. The treatment of ADHD comorbid with a SUD is complicated by risks for misuse and diversion of prescription stimulants” (Gray et al., 2018). The CPS makes no statement regarding mode of testing or frequency of testing.
Pain Management Best Practices Inter-Agency Task Force Report
The Pain Management Best Practices Inter-Agency Task Force recognizes the importance of screening and monitoring in pain management in identifying and reducing the risk of substance misuse, abuse, and overdose, as well as improving overall patient care. As such, they include a series of gaps in care and related recommendations regarding screening, including the following:
“GAP 1: Comprehensive screening and risk assessment of patients are time-consuming but vital for proper evaluation of their chronic pain conditions. Lack of sufficient compensation for time and payment for services have contributed to barriers
in best practices for opioid therapy.
• RECOMMENDATION 1A: Encourage CMS and private payers to provide sufficient compensation for time and payment for services to implement the various screening measures (e.g., extensive history taking, review of medical records, PDMP query, urine toxicology screenings, when clinically indicated). These are vital aspects of risk assessment and stratification for patients on opioids and other medications.
• RECOMMENDATION 1B: Consider referral to pain, mental health, and other specialists, including addiction medicine-trained physicians when high-risk patients are identified.
GAP 2: UDTs are not consistently used as part of the routine risk assessment for patients on opioids.
• RECOMMENDATION 2A: Use UDTs as part of the risk assessment tools prior to the initiation of opioid therapy and as a tool for reevaluating risk, using the clinical judgment of the treatment team.
• RECOMMENDATION 2B: Clinicians should educate patients on the use of UDTs and their role in identifying both appropriate and potentially inappropriate use” (PMFT, 2019).
References
- AACC. (2017). Using Clinical Laboratory Tests to Monitor Drug Therapy in Pain Management Patients. https://www.aacc.org/science-and-practice/practice-guidelines/using-clinical-laboratory-tests-to-monitor-drug-therapy-in-pain-management-patients
- AAFP. (2020). Clinical Preventive Service Recommendation Opioid Use Disorder (OUD): Screening. AAFP. Retrieved 1/26 from https://www.aafp.org/family-physician/patient-care/clinical-recommendations/all-clinical-recommendations/oud.html
- AAN. (2018). DIAGNOSTIC ASSESSMENT OF THE CHILD WITH STATUS EPILEPTICUS. https://www.aan.com/Guidelines/home/GuidelineDetail/234
- AAPM. (2013). Use of Opioids for the Treatment of Chronic Pain. https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/pain-management-toolkit/docs/use-of-opioids-for-the-treatment-of-chronic-pain.ashx
- AATOD. (2017). Guidelines for Addressing Benzodiazepine Use in Opioid Treatment Programs (OTPs). https://www.aatod.org/advocacy/policy-statements/guidelines-for-addressing-benzodiazepine-use-in-opioid-treatment-programs-otps-april-6-2017/
- ACOG. (2017). Opioid Use and Opioid Use Disorder in Pregnancy. https://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co711.pdf
- ALFA. (2019). https://www.cliawaived.com/web/items/pdf/ALF_03_3152_1_Panel_Drug_Test_Insert~493file1.pdf
- Algren, D. A., & Christian, M. R. (2015). Buyer Beware: Pitfalls in Toxicology Laboratory Testing. Mo Med, 112(3), 206-210. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6170116/
- Altunkaya, D., & Smith, R. N. (1990). Aberrant radioimmunoassay results for cannabinoids in urine. Forensic Sci Int, 47(3), 195-205.
- AMDG. (2015). Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf
- AMDG. (2018). Supplemental Guidance on Prescribing Opioids for Postoperative Pain. http://agencymeddirectors.wa.gov/Files/FinalSupBreeAMDGPostopPain091318wcover.pdf
- APA. (2016). THE AMERICAN PSYCHIATRIC ASSOCIATION PRACTICE GUIDELINES FOR THE PSYCHIATRIC EVALUATION OF ADULTS. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9780890426760
- Argoff, C. E., Alford, D. P., Fudin, J., Adler, J. A., Bair, M. J., Dart, R. C., Gandolfi, R., McCarberg, B. H., Stanos, S. P., Gudin, J. A., Polomano, R. C., & Webster, L. R. (2018). Rational Urine Drug Monitoring in Patients Receiving Opioids for Chronic Pain: Consensus Recommendations. Pain Med, 19(1), 97-117. https://doi.org/10.1093/pm/pnx285
- ASAM. (2013). Drug Testing: A White Paper of the American Society of Addiction Medicine (ASAM). https://www.cmm.com.au/files/uploads/resources/20170817102442drug-testing-a-white-paper-by-asam.pdf
- ASAM. (2017). Appropriate Use of Drug Testing in Clinical Addiction Medicine. file:///C:/Users/AHCS8430/OneDrive%20-%20AVALON%20HEALTH%20SERVICES,%20LLC/Desktop/ASAM%20Guidelines%20Appropriate%20Use%20of%20Drug%20Testing%20in%20Clinical%20Addiction%20Medicine.pdf
- Becker, W., & Starrels, J. (2022, 11/7/2022). Prescription drug misuse: Epidemiology, prevention, identification, and management. Wolters Kluwer. https://www.uptodate.com/contents/prescription-drug-misuse-epidemiology-prevention-identification-and-management
- Bertron, J. L., Seto, M., & Lindsley, C. W. (2018). DARK Classics in Chemical Neuroscience: Phencyclidine (PCP). ACS Chem Neurosci, 9(10), 2459-2474. https://doi.org/10.1021/acschemneuro.8b00266
- Blank, A., Hellstern, V., Schuster, D., Hartmann, M., Matthee, A. K., Burhenne, J., Haefeli, W. E., & Mikus, G. (2009). Efavirenz treatment and false-positive results in benzodiazepine screening tests. Clin Infect Dis, 48(12), 1787-1789. https://doi.org/10.1086/599109
- Böttcher, M., Lierheimer, S., Peschel, A., & Beck, O. (2019). Detection of heroin intake in patients in substitution treatment using oral fluid as specimen for drug testing. Drug Alcohol Depend, 198, 136-139. https://doi.org/10.1016/j.drugalcdep.2019.02.011
- Brahm, N. C., Yeager, L. L., Fox, M. D., Farmer, K. C., & Palmer, T. A. (2010). Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm, 67(16), 1344-1350. https://doi.org/10.2146/ajhp090477
- CDC. (2015, June 2015). Nationwide Trends. CDC. https://www.drugabuse.gov/publications/drugfacts/nationwide-trends
- CDC. (2017, 02/09/2017). Opioid Data Analysis and Resources. Centers for Disease Control and Prevention. Retrieved 10/01/2018 from https://www.cdc.gov/drugoverdose/data/analysis.html
- CDC. (2018a, December 2018). Monitoring the Future Survey: High School and Youth Trends. CDC. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends
- CDC. (2018b). Quality Improvement and Care Coordination: Implementing the CDC Guideline for Prescribing Opioids for Chronic Pain Retrieved 2/1/2021 from https://www.cdc.gov/drugoverdose/pdf/prescribing/CDC-DUIP-QualityImprovementAndCareCoordination-508.pdf
- CDC. (2019). Other Drugs. https://www.cdc.gov/drugoverdose/data/otherdrugs.html
- CDC. (2022a). Drug Overdose Deaths. https://www.cdc.gov/drugoverdose/deaths/index.html
- CDC. (2022b). U.S. Opioid Dispensing Rate Maps. https://www.cdc.gov/drugoverdose/rxrate-maps/index.html
- Chou, R., Fanciullo, G. J., Fine, P. G., Adler, J. A., Ballantyne, J. C., Davies, P., Donovan, M. I., Fishbain, D. A., Foley, K. M., Fudin, J., Gilson, A. M., Kelter, A., Mauskop, A., O'Connor, P. G., Passik, S. D., Pasternak, G. W., Portenoy, R. K., Rich, B. A., Roberts, R. G., . . . Miaskowski, C. (2009). Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain. The Journal of Pain, 10(2), 113-130.e122. https://doi.org/10.1016/j.jpain.2008.10.008
- Christo, P. J., Manchikanti, L., Ruan, X., Bottros, M., Hansen, H., Solanki, D. R., Jordan, A. E., & Colson, J. (2011). Urine drug testing in chronic pain. Pain Physician, 14(2), 123-143. https://www.painphysicianjournal.com/current/pdf?article=MTQ0NA%3D%3D&journal=60
- Chua, I., Petrides, A. K., Schiff, G. D., Ransohoff, J. R., Kantartjis, M., Streid, J., Demetriou, C. A., & Melanson, S. E. F. (2020). Provider Misinterpretation, Documentation, and Follow-Up of Definitive Urine Drug Testing Results. J Gen Intern Med, 35(1), 283-290. https://doi.org/10.1007/s11606-019-05514-5
- Cone, E. J., Bigelow, G. E., Herrmann, E. S., Mitchell, J. M., LoDico, C., Flegel, R., & Vandrey, R. (2015). Non-smoker exposure to secondhand cannabis smoke. I. Urine screening and confirmation results. J Anal Toxicol, 39(1), 1-12. https://doi.org/10.1093/jat/bku116
- Couto, J. E., Webster, L., Romney, M. C., Leider, H. L., & Linden, A. (2009). Use of an algorithm applied to urine drug screening to assess adherence to an oxycontin regimen. J Opioid Manag, 5(6), 359-364.
- Couto, J. E., Webster, L., Romney, M. C., Leider, H. L., & Linden, A. (2011). Use of an algorithm applied to urine drug screening to assess adherence to a hydrocodone regimen. J Clin Pharm Ther, 36(2), 200-207. https://doi.org/10.1111/j.1365-2710.2010.01236.x
- DOD. (2017). VA/DoD CLINICAL PRACTICE GUIDELINE FOR OPIOID THERAPY FOR CHRONIC PAIN. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf
- DOD. (2018). VA/DOD CLINICAL PRACTICE GUIDELINE FOR THE MANAGEMENT OF PREGNANCY. https://www.healthquality.va.gov/guidelines/WH/up/VADoDPregnancyCPG4102018.pdf
- Dowell, D., Haegerich, T. M., & Chou, R. (2016). CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep, 65(1), 1-49. https://doi.org/10.15585/mmwr.rr6501e1
- Drake, L. R., & Scott, P. J. H. (2018). DARK Classics in Chemical Neuroscience: Cocaine. ACS Chem Neurosci, 9(10), 2358-2372. https://doi.org/10.1021/acschemneuro.8b00117
- DVA, & DOD. (2015). VA/DoD CLINICAL PRACTICE GUIDELINE FOR THE MANAGEMENT OF SUBSTANCE USE DISORDERS Retrieved 1/28/2021 from https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
- DWD. (2013). Chronic Opioid Clinical Management Guidelines for Wisconsin Worker’s Compensation Patient Care. Dejpartment of Workforce Development, State of Wisconsin. https://dwd.wisconsin.gov/wc/medical/pdf/CHRONIC%20OPIOID%20CLINICAL%20MANAGEMENT%20GUIDELINES%20.pdf
- Eaton, K., & Lyman, G. (2022, 10/21/2022). Dosing of anticancer agents in adults. Wolters Kluwer. https://www.uptodate.com/contents/dosing-of-anticancer-agents-in-adults
- Eskridge, K. D., & Guthrie, S. K. (1997). Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy, 17(3), 497-510.
- Farkas, A., Lipanot, K., & Sherman, K. (2021). Routine Laboratory Screening for Acetaminophen and Salicylate Ingestion in Preadmission Psychiatric Patients Is Unnecessary. Ann Emerg Med, 77(6), 604-612. https://doi.org/10.1016/j.annemergmed.2021.01.027
- FDA. (2018). DRI Cocaine Metabolite Assay. https://www.accessdata.fda.gov/cdrh_docs/pdf18/K181499.pdf
- FDA. (2019). 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE https://www.accessdata.fda.gov/cdrh_docs/reviews/K112395.pdf
- FSMB. (2017). Guidelines for the Chronic Use of Opioid Analgesics. Federation of State Medical Boards. Retrieved 10/01/2018 from https://www.fsmb.org/globalassets/advocacy/policies/opioid_guidelines_as_adopted_april-2017_final.pdf
- Fucci, N. (2012). False positive results for amphetamine in urine of a patient with diabetes mellitus. Forensic Sci Int, 223(1-3), e60. https://doi.org/10.1016/j.forsciint.2012.08.010
- Grant, C. N., & Bélanger, R. E. (2017). Position Statement: Cannabis and Canada’s children and youth. Pediatric Child Health, 22(2), 98-102. https://www.cps.ca/en/documents/position/cannabis-children-and-youth
- Gray, C., Korczak, D., Andrews, D., & Bélanger, S. A. (2018). ADHD in children and youth: Part 1—Etiology, diagnosis, and comorbidity. Paediatrics & Child Health, 23(7), 447-453. https://doi.org/10.1093/pch/pxy109
- Greller, H., & Gupta, A. (2020, 10/20/2020). Benzodiazepine poisoning and withdrawal. Wolters Kluwer. Retrieved 1/28/2021 from https://www.uptodate.com/contents/benzodiazepine-poisoning-and-withdrawal?topicRef=13846&source=see_link#H9
- Herrmann, E. S., Cone, E. J., Mitchell, J. M., Bigelow, G. E., LoDico, C., Flegel, R., & Vandrey, R. (2015). Non-smoker exposure to secondhand cannabis smoke II: Effect of room ventilation on the physiological, subjective, and behavioral/cognitive effects. Drug Alcohol Depend, 151, 194-202. https://doi.org/10.1016/j.drugalcdep.2015.03.019
- HHS. (2017). New HHS Guidelines for Drug Testing Panels Effective 10/1/17. https://www.federalregister.gov/documents/2017/01/23/2017-00979/mandatory-guidelines-for-federal-workplace-drug-testing-programs#h-114
- Hoffman, R. (2022, 9/21/2022). Testing for drugs of abuse (DOAs). Wolters Kluwer. https://www.uptodate.com/contents/testing-for-drugs-of-abuse-doas
- Jamison, R. N., Ross, E. L., Michna, E., Chen, L. Q., Holcomb, C., & Wasan, A. D. (2010). Substance misuse treatment for high-risk chronic pain patients on opioid therapy: a randomized trial. Pain, 150(3), 390-400. https://doi.org/10.1016/j.pain.2010.02.033
- Jannetto, P., Bratanow, N., Clark, W., Hamill-Ruth, R., Hammett-Stabler, C., Huestis, M., Kassed, C., McMillin, G., Melanson, S., & Langman, L. (2017). Using Clinical Laboratory Tests to Monitor Drug Therapy in Pain Management Patients. AACC. Retrieved 10/01/2018 from https://www.aacc.org/science-and-practice/practice-guidelines/using-clinical-laboratory-tests-to-monitor-drug-therapy-in-pain-management-patients
- Jannetto, P. J., & Langman, L. J. (2018). Using Clinical Laboratory Tests to Monitor Drug Therapy in Pain Management Patients. The Journal of Applied Laboratory Medicine: An AACC Publication, 2(4), 471-472. https://doi.org/10.1373/jalm.2017.025304
- Jarvis, M., Williams, J., Hurford, M., Lindsay, D., Lincoln, P., Giles, L., Luongo, P., & Safarian, T. (2017). Appropriate Use of Drug Testing in Clinical Addiction Medicine. J Addict Med, 11(3), 163-173. https://doi.org/10.1097/adm.0000000000000323
- Jones, C. M., Mack, K. A., & Paulozzi, L. J. (2013). Pharmaceutical overdose deaths, United States, 2010. Jama, 309(7), 657-659. https://doi.org/10.1001/jama.2013.272
- Jones, J. (2016, 07/28/2016). Clinical vs. Forensic: The Differences Cost More Than Just Money. United States Drug Testing Laboratories. Retrieved 02/05/2019 from http://www.usdtl.com/media/mediaarticles/clinical-vs-forensic-the-differences-cost-more-than-just-money
- Kale, N. (2019). Urine Drug Tests: Ordering and Interpreting Results. Am Fam Physician, 99(1), 33-39. https://www.aafp.org/afp/2019/0101/p33.pdf
- Kampman, K. (2022, 7/11/2022). Approach to treatment of stimulant use disorder in adults. Retrieved 1/28/2021 from https://www.uptodate.com/contents/approach-to-treatment-of-stimulant-use-disorder-in-adults
- Kandel, D. B., Hu, M. C., Griesler, P., & Wall, M. (2017). Increases from 2002 to 2015 in prescription opioid overdose deaths in combination with other substances. Drug Alcohol Depend, 178, 501-511. https://doi.org/10.1016/j.drugalcdep.2017.05.047
- Katz, N. P., Sherburne, S., Beach, M., Rose, R. J., Vielguth, J., Bradley, J., & Fanciullo, G. J. (2003). Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg, 97(4), 1097-1102, table of contents.
- Katzman, M. A., Bleau, P., Blier, P., Chokka, P., Kjernisted, K., Van Ameringen, M., Canadian Anxiety Guidelines Initiative Group on behalf of the Anxiety Disorders Association of Canada/Association Canadienne des troubles, a., McGill, U., Antony, M. M., Bouchard, S., Brunet, A., Flament, M., Grigoriadis, S., Mendlowitz, S., O'Connor, K., Rabheru, K., Richter, P. M. A., Robichaud, M., & Walker, J. R. (2014). Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC psychiatry, 14 Suppl 1(Suppl 1), S1-S1. https://doi.org/10.1186/1471-244X-14-S1-S1
- Kell, M. J. (1994). Utilization of plasma and urine methadone concentrations to optimize treatment in maintenance clinics: I. Measurement techniques for a clinical setting. J Addict Dis, 13(1), 5-26. https://doi.org/10.1300/J069v13n01_02
- Knezevic, N. N., Khan, O. M., Beiranvand, A., & Candido, K. D. (2017). Repeated Quantitative Urine Toxicology Analysis May Improve Chronic Pain Patient Compliance with Opioid Therapy. Pain Physician, 20(2s), S135-s145.
- Krasowski, M. D., McMillin, G. A., Melanson, S. E. F., Dizon, A., Magnani, B., & Snozek, C. L. H. (2020). Interpretation and Utility of Drug of Abuse Screening Immunoassays: Insights From Laboratory Drug Testing Proficiency Surveys. Arch Pathol Lab Med, 144(2), 177-184. https://doi.org/10.5858/arpa.2018-0562-CP
- Langman, L. J., & Jannetto, P. J. (2018). Using Clinical Laboratory Tests to Monitor Drug Therapy in Pain Management Patients. https://www.aacc.org/-/media/Files/Science-and-Practice/Practice-Guidelines/Pain-Management/LMPGPain-Management20171220.pdf?la=en&hash=19670524407619F78999AB60731A24CB4901939D
- Levine, B. S., & Smith, M. L. (1990). Effects of diphenhydramine on immunoassays of phencyclidine in urine. Clin Chem, 36(6), 1258.
- Ly, B. T., Thornton, S. L., Buono, C., Stone, J. A., & Wu, A. H. (2012). False-positive urine phencyclidine immunoassay screen result caused by interference by tramadol and its metabolites. Ann Emerg Med, 59(6), 545-547. https://doi.org/10.1016/j.annemergmed.2011.08.013
- Manchikanti, L., Abdi, S., Atluri, S., Balog, C. C., Benyamin, R. M., Boswell, M. V., Brown, K. R., Bruel, B. M., Bryce, D. A., Burks, P. A., Burton, A. W., Calodney, A. K., Caraway, D. L., Cash, K. A., Christo, P. J., Damron, K. S., Datta, S., Deer, T. R., Diwan, S., . . . Wargo, B. W. (2012). American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2--guidance. Pain Physician, 15(3 Suppl), S67-116.
- Manchikanti, L., Kaye, A. M., Knezevic, N. N., McAnally, H., Slavin, K., Trescot, A. M., Blank, S., Pampati, V., Abdi, S., Grider, J. S., Kaye, A. D., Manchikanti, K. N., Cordner, H., Gharibo, C. G., Harned, M. E., Albers, S. L., Atluri, S., Aydin, S. M., Bakshi, S., . . . Hirsch, J. A. (2017). Responsible, Safe, and Effective Prescription of Opioids for Chronic Non-Cancer Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician, 20(2s), S3-s92.
- Manchikanti, L., Malla, Y., Wargo, B. W., Cash, K. A., Pampati, V., Damron, K. S., McManus, C. D., & Brandon, D. E. (2010). Protocol for accuracy of point of care (POC) or in-office urine drug testing (immunoassay) in chronic pain patients: a prospective analysis of immunoassay and liquid chromatography tandem mass spectometry (LC/MS/MS). Pain Physician, 13(1), E1-e22. https://www.painphysicianjournal.com/current/pdf?article=MTMwMg%3D%3D&journal=53
- McClellan, J., & Stock, S. (2013). Practice Parameter for the Assessment and Treatment of Children and Adolescents With Schizophrenia. Journal of the American Academy of Child & Adolescent Psychiatry, 52(9), 976-990. https://doi.org/10.1016/j.jaac.2013.02.008
- McEvoy, J., Millet, R. A., Dretchen, K., Morris, A. A., Corwin, M. J., & Buckley, P. (2014). Quantitative levels of aripiprazole parent drug and metabolites in urine. Psychopharmacology (Berl), 231(23), 4421-4428. https://doi.org/10.1007/s00213-014-3781-1
- Michna, E., Jamison, R. N., Pham, L. D., Ross, E. L., Janfaza, D., Nedeljkovic, S. S., Narang, S., Palombi, D., & Wasan, A. D. (2007). Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin J Pain, 23(2), 173-179. https://doi.org/10.1097/AJP.0b013e31802b4f95
- Microgenics. (2015). CEDIA(r) Phencyclidine (PCP) Assay. Microgenics Corporation. http://tools.thermofisher.com/content/sfs/manuals/10007400-CEDIA-Phencyclidine-PCP-Assay-EN.pdf
- Microgenics. (2016). DRI(r) Amphetamines Assay. Microgenics Corporation. Retrieved 02/12/2019 from http://tools.thermofisher.com/content/sfs/manuals/0138-DRI-Amphetamines-Assay-EN.pdf
- Moeller, K. E., Kissack, J. C., Atayee, R. S., & Lee, K. C. (2017). Clinical Interpretation of Urine Drug Tests: What Clinicians Need to Know About Urine Drug Screens. Mayo Clin Proc, 92(5), 774-796. https://doi.org/10.1016/j.mayocp.2016.12.007
- Nafziger, A. N., & Bertino, J. S., Jr. (2009). Utility and application of urine drug testing in chronic pain management with opioids. Clin J Pain, 25(1), 73-79. https://doi.org/10.1097/AJP.0b013e31817e13cc
- National Center for Drug Abuse Statistics. (2022). Drug Abust Statistics https://drugabusestatistics.org/
- Nelson, L., & Odujebe, O. (2022, 6/7/2022). Cocaine: Acute intoxication. Retrieved 1/28/2021 from https://www.uptodate.com/contents/cocaine-acute-intoxication
- NICE. (2018, April 2018). Epilepsies: diagnosis and management. https://www.nice.org.uk/guidance/cg137/chapter/1-Guidance
- NICE. (2020). Epilepsies: diagnosis and management. https://www.nice.org.uk/guidance/cg137/chapter/1-Guidance
- Nuckols, T. K., Anderson, L., Popescu, I., Diamant, A. L., Doyle, B., Di Capua, P., & Chou, R. (2014). Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med, 160(1), 38-47. https://doi.org/10.7326/0003-4819-160-1-201401070-00732
- OASAS. (2021). Guidance on Toxicology Use in OASAS Certified Programs. https://oasas.ny.gov/system/files/documents/2021/11/oasas-toxicology-guidance_0.pdf
- Ordean, A., Wong, S., & Graves, L. (2017). No. 349-Substance Use in Pregnancy. J Obstet Gynaecol Can, 39(10), 922-937.e922. https://doi.org/10.1016/j.jogc.2017.04.028
- Owen, G. T., Burton, A. W., Schade, C. M., & Passik, S. (2012). Urine drug testing: current recommendations and best practices. Pain Physician, 15(3 Suppl), Es119-133.
- Owusu Obeng, A., Hamadeh, I., & Smith, M. (2017). Review of Opioid Pharmacogenetics and Considerations for Pain Management. Pharmacotherapy, 37(9), 1105-1121. https://doi.org/10.1002/phar.1986
- Palamar, J. J., Le, A., Guarino, H., & Mateu-Gelabert, P. (2019). A comparison of the utility of urine- and hair testing in detecting self-reported drug use among young adult opioid users. Drug Alcohol Depend, 200, 161-167. https://doi.org/10.1016/j.drugalcdep.2019.04.008
- Pesce, A., Krock, K., Ritz, D., Cua, A., Thomas, R., & Nickley, J. (2018). Observations on 6-MAM (6-Monoacetylmorphine) in Urine. J Clin Toxicol, 8(393), 2161-0495.1000393.
- Pesce, A., West, C., Egan City, K., & Strickland, J. (2012). Interpretation of urine drug testing in pain patients. Pain Med, 13(7), 868-885. https://doi.org/10.1111/j.1526-4637.2012.01350.x
- Phan, H. M., Yoshizuka, K., Murry, D. J., & Perry, P. J. (2012). Drug testing in the workplace. Pharmacotherapy, 32(7), 649-656. https://doi.org/10.1002/j.1875-9114.2011.01089.x
- PMFT. (2019). PAIN MANAGEMENT BEST PRACTICES INTER-AGENCY TASK FORCE REPORT
- Updates, Gaps, Inconsistencies, and Recommendations
- PAIN MANAGEMENT
- FINAL REPORT https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
- Rengarajan, A., & Mullins, M. E. (2013). How often do false-positive phencyclidine urine screens occur with use of common medications? Clin Toxicol (Phila), 51(6), 493-496. https://doi.org/10.3109/15563650.2013.801982
- Rollins, D. E., Jennison, T. A., & Jones, G. (1990). Investigation of interference by nonsteroidal anti-inflammatory drugs in urine tests for abused drugs. Clin Chem, 36(4), 602-606.
- SAMHSA. (2007). Guidelines for the Accreditation of Opioid Treatment Programs https://www.samhsa.gov/sites/default/files/programs_campaigns/medication_assisted/2007-otp-accreditation-guidelines.pdf
- SAMHSA. (2015). FEDERAL GUIDELINES FOR OPIOID TREATMENT PROGRAMS. https://store.samhsa.gov/system/files/pep15-fedguideotp.pdf
- SAMHSA. (2017). Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf
- SAMHSA. (2020). Mandatory Guidelines for Federal Workplace Drug Testing Programs— Oral/Fluid https://www.samhsa.gov/sites/default/files/programs_campaigns/division_workplace_programs/final-mg-oral-fluid.pdf
- SAMHSA. (2022). Mandatory Guidelines for Federal Workplace Drug Testing Programs. Federal Register. https://www.federalregister.gov/documents/2022/04/07/2022-06884/mandatory-guidelines-for-federal-workplace-drug-testing-programs#sectno-reference-2.2
- Smith, P. E., & McBride, A. (1999). Illicit drugs and seizures. Seizure, 8(8), 441-443. https://doi.org/10.1053/seiz.1999.0346
- Snyder, M. L., Fantz, C. R., & Melanson, S. (2017). Immunoassay-Based Drug Tests Are Inadequately Sensitive for Medication Compliance Monitoring in Patients Treated for Chronic Pain. Pain Physician, 20(2s), Se1-se9. https://www.painphysicianjournal.com/current/pdf?article=NDIwNw%3D%3D&journal=103
- Starrels, J. L., Becker, W. C., Alford, D. P., Kapoor, A., Williams, A. R., & Turner, B. J. (2010). Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med, 152(11), 712-720. https://doi.org/10.7326/0003-4819-152-11-201006010-00004
- Trescot, A. M., Datta, S., Lee, M., & Hansen, H. (2008). Opioid pharmacology. Pain Physician, 11(2 Suppl), S133-153.
- Vohra, V., Marraffa, J. M., Wojcik, S. M., & Eggleston, W. (2019). An assessment of urine THC immunoassay in healthy volunteers receiving an oral proton-pump inhibitor. Clin Toxicol (Phila), 1-3. https://doi.org/10.1080/15563650.2019.1662917
- Vopat, M. L., Messamore, W. G., Trent, J. J., Schmanke, K. E., Zackula, R., Yang, S. Y., & Bhargava, T. (2020). Urine Screening for Opioid and Illicit Drugs in the Total Joint Arthroplasty Population. Kans J Med, 13, 71-76.
- Weaver, M. F. (2015). Prescription Sedative Misuse and Abuse. Yale J Biol Med, 88(3), 247-256. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4553644/
- WFSBP. (2015). World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia Part 3: Update 2015 Management of special circumstances: Depression, Suicidality, substance use disorders and pregnancy and lactation http://www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Hasan_et_al__2015_.pdf
- WHO. (2016). mhGAP Intervention Guide. https://apps.who.int/iris/bitstream/handle/10665/250239/9789241549790-eng.pdf?sequence=1
- Wilfong, A. (2021, 11/23/2021). Management of convulsive status epilepticus in children. https://www.uptodate.com/contents/management-of-convulsive-status-epilepticus-in-children
- Wondfo. (2020). DRUG TESTS STRIP. http://wondfousa.com/test/strip/
- Wong, S., Ordean, A., & Kahan, M. (2011). Substance use in pregnancy. J Obstet Gynaecol Can, 33(4), 367-384. https://doi.org/10.1016/s1701-2163(16)34855-1
- Xlar. (2002). THC One Step Marijuana Test Strip Package Insert. xlar.com/I/THC-STRIP-2.pdf
Coding Section
| Code | Number | Code Description |
| CPT | 80305 | Drug tests(s), presumptive, any number of drug classes; any number of devices or procedures, (e.g., immunoassay) capable of being read by direct optical observation only (e.g., dipsticks, cups, cards, cartridges), includes sample validation when performed, per date of service |
| 80306 | Drug tests(s), presumptive, any number of drug classes; any number of devices or procedures, (e.g., immunoassay) read by instrument-assisted direct optical observation (e.g., dipsticks, cups, cards, cartridges), includes sample validation when performed, per date of service | |
| 80307 | Drug test(s), presumptive, any number of drug classes, any number of devices or procedures, by instrument chemistry analyzers (e.g., utilizing immunoassay [e.g., EIA, ELISA, EMIT, FPIA, IA, KIMS, RIA]), chromatography (e.g., GC, HPLC), and mass spectrometry either with or without chromatography, (e.g., DART, DESI, GC-MS, GC-MS/MS, LC-MS, LC-MS/MS, LDTD, MALDI, TOF) includes sample validation when performed, per date of service | |
| 0007U | Drug test(s), presumptive, with definitive confirmation of positive results, any number of drug classes, urine, includes specimen verification including DNA authentication in comparison to buccal DNA, per date of service | |
| 0011U | Prescription drug monitoring, evaluation of drugs present by LC-MS/MS, using oral fluid, reported as a comparison to an estimated steady-state range, per date of service including all drug compounds and metabolites | |
| 0051U | Prescription drug monitoring, evaluation of drugs present by LC-MS/MS, urine, 31 drug panel, reported as quantitative results, detected or not detected, per date of service | |
| 0054U | Prescription drug monitoring, 14 or more classes of drugs and substances, definitive tandem mass spectrometry with chromatography, capillary blood, quantitative report with therapeutic and toxic ranges, including steady-state range for the prescribed dose when detected, per date of service | |
| 0079U | Comparative DNA analysis using multiple selected single-nucleotide polymorphisms (SNPs), urine and buccal DNA, for specimen identity verificationProprietary test: ToxLok™Lab/Manufacturer: InSource Diagnostics | |
| 0082U | Drug test(s), definitive, 90 or more drugs or substances, definitive chromatography with mass spectrometry, and presumptive, any number of drug classes, by instrument chemistry analyzer (utilizing immunoassay), urine, report of presence or absence of each drug, drug metabolite or substance with description and severity of significant interactions per date of serviceProprietary test: NextGen Precision™ TestingLab/Manufacturer: Precision Diagnostics LBN Precision Toxicology, LLC | |
| 0093U | Prescription drug monitoring, evaluation of 65 common drugs by LC-MS/MS, urine, each drug reported detected or not detectedProprietary test: ComplyRXLab/Manufacturer: Claro Labs | |
| 0143U | Drug assay, definitive, 120 or more drugs or metabolites, urine, quantitative liquid chromatography with tandem mass spectrometry (LC-MS/MS) using multiple reaction monitoring (MRM), with drug or metabolite description, comments including sample validation, per date of serviceProprietary test: CareViewRxLab/Manufacturer: Newstar Medical Laboratories, LLC | |
| 0144U | Drug assay, definitive, 160 or more drugs or metabolites, urine, quantitative liquid chromatography with tandem mass spectrometry (LC-MS/MS) using multiple reaction monitoring (MRM), with drug or metabolite description, comments including sample validation, per date of serviceProprietary test: CareViewRx PlusLab/Manufacturer: Newstar Medical Laboratories, LLC | |
| 0145U | Drug assay, definitive, 65 or more drugs or metabolites, urine, quantitative liquid chromatography with tandem mass spectrometry (LC-MS/MS) using multiple reaction monitoring (MRM), with drug or metabolite description, comments including sample validation, per date of serviceProprietary test: PainViewRxLab/Manufacturer: Newstar Medical Laboratories, LLC | |
| 0146U | Drug assay, definitive, 80 or more drugs or metabolites, urine, by quantitative liquid chromatography with tandem mass spectrometry (LC-MS/MS) using multiple reaction monitoring (MRM), with drug or metabolite description, comments including sample validation, per date of serviceProprietary test: PainViewRx PlusLab/Manufacturer: Newstar Medical Laboratories, LLC | |
| 0147U | Drug assay, definitive, 85 or more drugs or metabolites, urine, quantitative liquid chromatography with tandem mass spectrometry (LC-MS/MS) using multiple reaction monitoring (MRM), with drug or metabolite description, comments including sample validation, per date of serviceProprietary test: RiskViewRxLab/Manufacturer: Newstar Medical Laboratories, LLC | |
| 0148U | Drug assay, definitive, 100 or more drugs or metabolites, urine, quantitative liquid chromatography with tandem mass spectrometry (LC-MS/MS) using multiple reaction monitoring (MRM), with drug or metabolite description, comments including sample validation, per date of serviceProprietary test: RiskViewRx PlusLab/Manufacturer: Newstar Medical Laboratories, LLC | |
| 0149U | Drug assay, definitive, 60 or more drugs or metabolites, urine, quantitative liquid chromatography with tandem mass spectrometry (LC-MS/MS) using multiple reaction monitoring (MRM), with drug or metabolite description, comments including sample validation, per date of serviceProprietary test: PsychViewRxLab/Manufacturer: Newstar Medical Laboratories, LLC | |
| 0150U | Drug assay, definitive, 120 or more drugs or metabolites, urine, quantitative liquid chromatography with tandem mass spectrometry (LC-MS/MS) using multiple reaction monitoring (MRM), with drug or metabolite description, comments including sample validation, per date of serviceProprietary test: PsychViewRx PlusLab/Manufacturer: Newstar Medical Laboratories, LLC | |
| 0227U | Drug assay, presumptive, 30 or more drugs or metabolites, urine, liquid chromatography with tandem mass spectrometry (LC-MS/MS) using multiple reaction monitoring (MRM), with drug or metabolite description, includes sample validationProprietary Test: Comprehensive ScreenLab/Manufacturer: Aspenti Health | |
| 0328U | Drug assay, definitive, 120 or more drugs and metabolites, urine, quantitative liquid chromatography with tandem mass spectrometry (LC-MS/MS), includes specimen validity and algorithmic analysis describing drug or metabolite and presence or absence of risks for a significant patient-adverse event, per date of service Proprietary test: CareView360 Lab/Manufacturer: Newstar Medical Laboratories, LLC |
|
| HCPCS | G0480 | Drug test(s), definitive, utilizing drug identification methods able to identify individual drugs and distinguish between structural isomers (but not necessarily stereoisomers), including, but not limited to GC/MS (any type, single or tandem) and LC/MS (any type, single or tandem and excluding immunoassays [e.g., IA, EIA, ELISA, EMIT, FPIA] and enzymatic methods [e.g., alcohol dehydrogenase]); qualitative or quantitative, all sources, includes specimen validity testing, per day, 1-7 drug class(es), including metabolite(s) if performed |
| G0481 | Drug test(s), definitive, utilizing drug identification methods able to identify individual drugs and distinguish between structural isomers (but not necessarily stereoisomers), including, but not limited to GC/MS (any type, single or tandem) and LC/MS (any type, single or tandem and excluding immunoassays [e.g., IA, EIA, ELISA, EMIT, FPIA] and enzymatic methods [e.g., alcohol dehydrogenase]); qualitative or quantitative, all sources, includes specimen validity testing, per day, 8 – 14 drug class(es), including metabolite(s) if performed | |
| G0482 | Drug test(s), definitive, utilizing drug identification methods able to identify individual drugs and distinguish between structural isomers (but not necessarily stereoisomers), including, but not limited to GC/MS (any type, single or tandem) and LC/MS (any type, single or tandem and excluding immunoassays [e.g., IA, EIA, ELISA, EMIT, FPIA] and enzymatic methods [e.g., alcohol dehydrogenase]); qualitative or quantitative, all sources, includes specimen validity testing, per day, 15 – 21 drug class(es), including metabolite(s) if performed.) | |
| G0483 | Drug test(s), definitive, utilizing drug identification methods able to identify individual drugs and distinguish between structural isomers (but not necessarily stereoisomers), including, but not limited to GC/MS (any type, single or tandem) and LC/MS (any type, single or tandem and excluding immunoassays [e.g., IA, EIA, ELISA, EMIT, FPIA] and enzymatic methods [e.g., alcohol dehydrogenase]); qualitative or quantitative, all sources, includes specimen validity testing, per day, 22 or more drug class(es), including metabolite(s) if performed | |
| G0659 | Drug test(s), definitive, utilizing drug identification methods able to identify individual drugs and distinguish between structural isomers (but not necessarily stereoisomers), including but not limited to GC/MS (any type, single or tandem) and LC/MS (any type, single or tandem), excluding immunoassays (e.g., IA, EIA, ELISA, EMIT, FPIA) and enzymatic methods (e.g., alcohol dehydrogenase), performed without method or drug-specific calibration, without matrix-matched quality control material, or without use of stable isotope or other universally recognized internal standard(s) for each drug, drug metabolite or drug class per specimen; qualitative or quantitative, all sources, includes specimen validity testing, per day, any number of drug classes |
Procedure and diagnosis codes on Medical Guideline documents are included only as a general reference tool for each guideline. They may not be all-inclusive.
This medical guideline was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, Blue Cross Blue Shield Association technology assessment program (TEC) and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2016 Forward
| 05/04/2023 | Annual review, updating policy for clarity and consistency, no change to intent. Updating rationale and references. |
| 08/22/2022 | Interim Review to correct misformatting in the rationale. No other changes made. |
| 06/15/2022 | Adding code 0328U |
|
04/12/2022 |
Annual review, no change to policy intent, but, some grammatical updates were made for clarity. Also updating rationale, references and adding table of terminology. |
|
09/22/2021 |
Correcting a typo in policy section. No change to intent to policy. |
|
07/29/2021 |
Interim review, updating policy verbiage for clarity and specificity. |
|
04/01/2021 |
Annual review, no change to policy intent. Updating rationale and references. |
|
10/01/2020 |
Interim review, adding "or oral fluid" to the statement regarding specimens for members with chronic renal failure. No other changes made. |
|
04/29/2020 |
Major revision for clarity and specificity including title change. |
|
08/06/2018 |
Annual review, adding verbiage regarding testing in pregnancy. Updating review date. Otherwise, no changes made. |
|
12/05/2017 |
Updating policy with 2018 coding. No other changes made to policy. |
|
08/03/2017 |
Annual review, expanding criteria for presumptive and definitive testing. Updating coding to mirror code ranges 80305-80307 with G0477-G0479. No other changes made. |
|
04/25/2017 |
Updated category to Laboratory. No other changes. |
|
12/05/2016 |
Updated coding section with 2017 codes. |
|
03/01/2016 |
NEW POLICY |